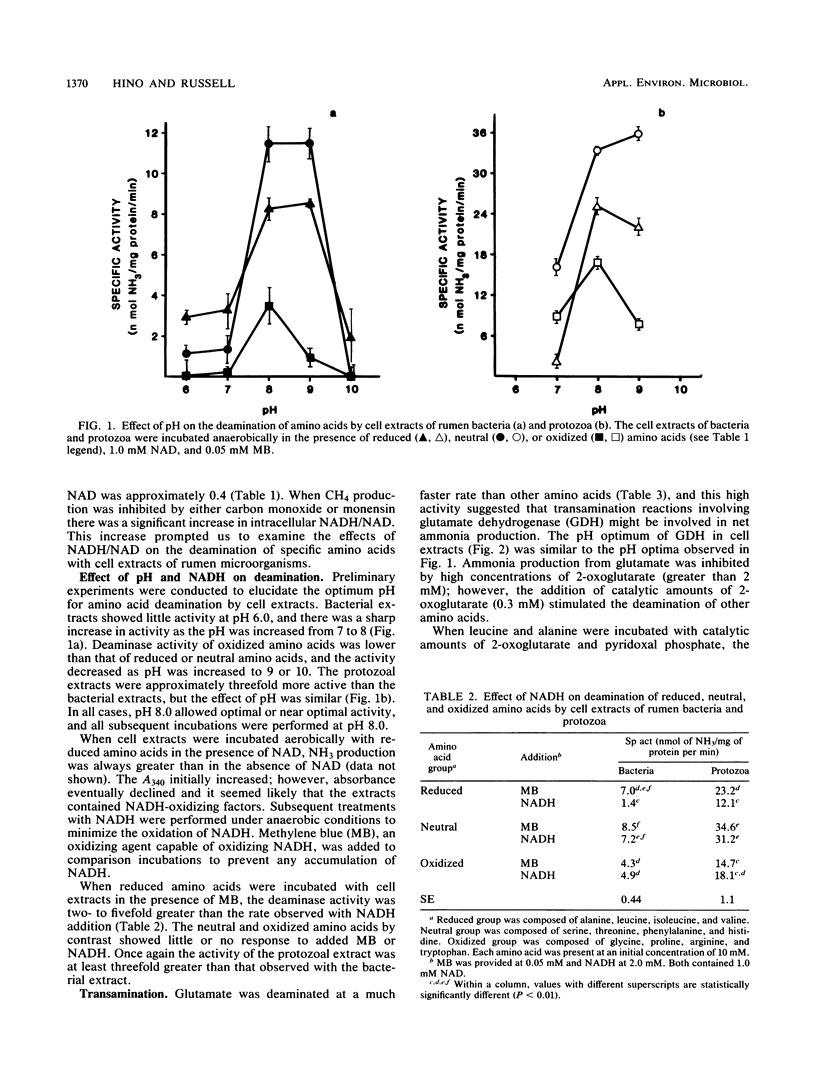
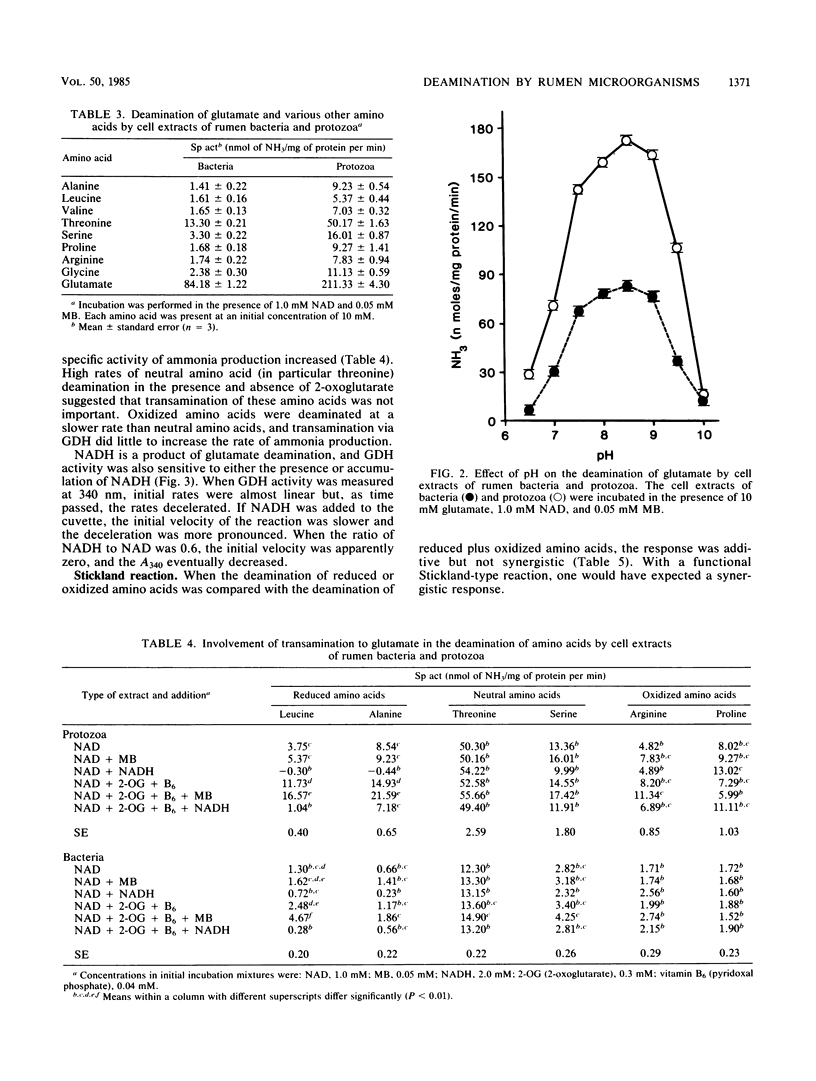
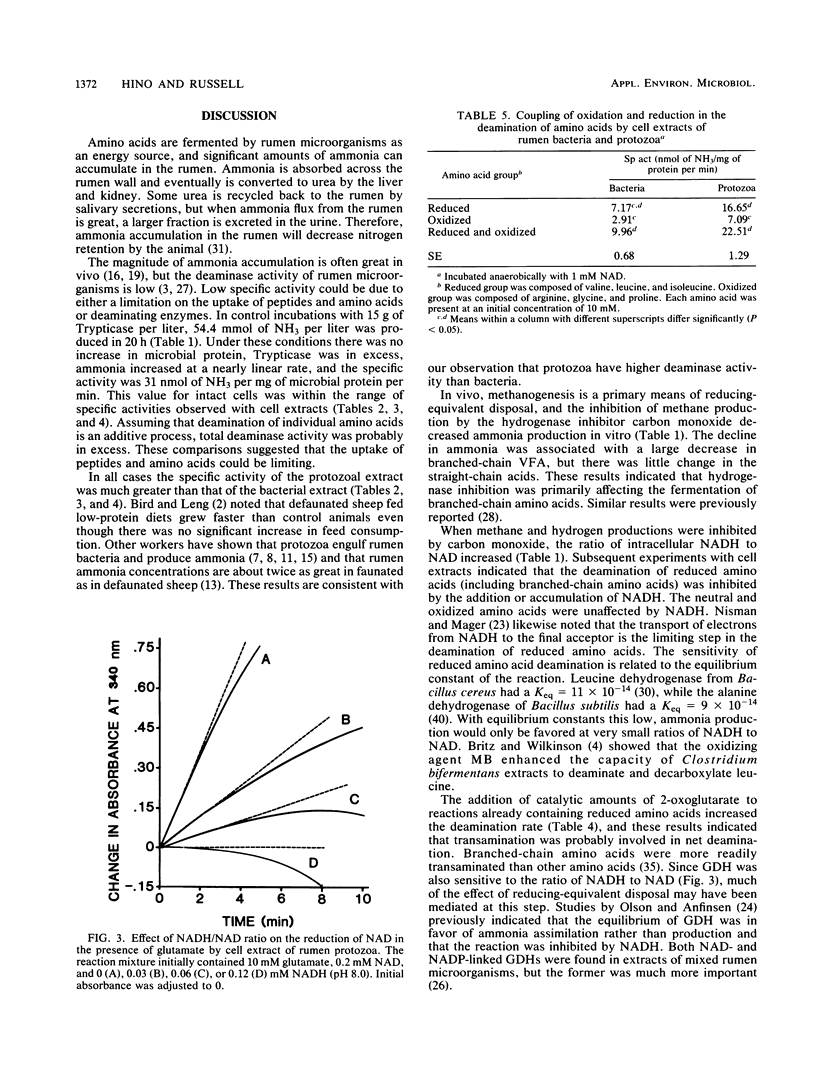
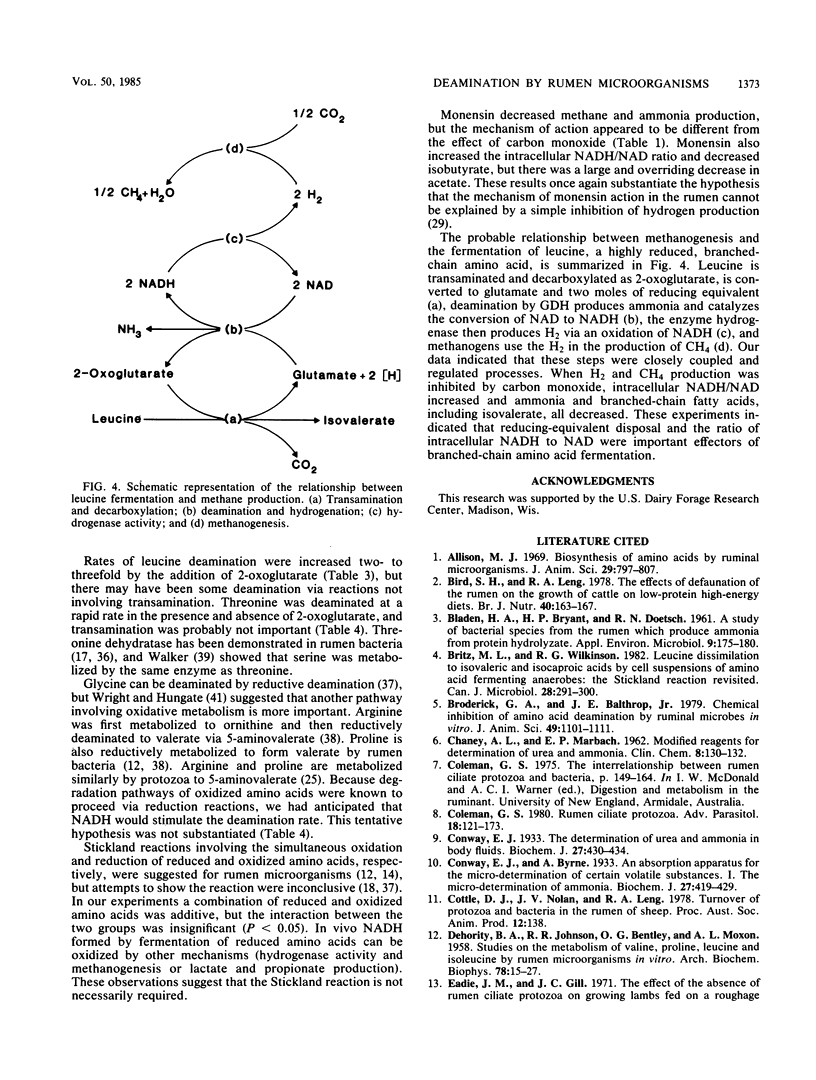
Abstract
When mixed rumen microorganisms were incubated in media containing the amino acid source Trypticase, both monensin and carbon monoxide (a hydrogenase inhibitor) decreased methane formation and amino acid fermentation. Both of the methane inhibitors caused a significant increase in the ratio of intracellular NADH to NAD. Studies with cell extracts of rumen bacteria and protozoa indicated that the ratio of NADH to NAD had a marked effect on the deamination of reduced amino acids, in particular branched-chain amino acids. Deamination was inhibited by the addition of NADH and was stimulated by methylene blue, an agent that oxidizes NADH. Neutral and oxidized amino acids were unaffected by NADH. The addition of small amounts of 2-oxoglutarate greatly enhanced the deamination of branched-chain amino acids and indicated that transamination via glutamate dehydrogenase was important. Formation of ammonia from glutamate was likewise inhibited by NADH. These experiments indicated that reducing-equivalent disposal and intracellular NADH/NAD ratio were important effectors of branched-chain amino acid fermentation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison M. J. Biosynthesis of amono acids by ruminal microorganisms. J Anim Sci. 1969 Nov;29(5):797–807. doi: 10.2527/jas1969.295797x. [DOI] [PubMed] [Google Scholar]
- Bird S. H., Leng R. A. The effects of defaunation of the rumen on the growth of cattle on low-protein high-energy diets. Br J Nutr. 1978 Jul;40(1):163–167. doi: 10.1079/bjn19780108. [DOI] [PubMed] [Google Scholar]
- Bladen H. A., Bryant M. P., Doetsch R. N. A Study of Bacterial Species from the Rumen Which Produce Ammonia from Protein Hydrolyzate. Appl Microbiol. 1961 Mar;9(2):175–180. doi: 10.1128/am.9.2.175-180.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britz M. L., Wilkinson R. G. Leucine dissimilation to isovaleric and isocaproic acids by cell suspensions of amino acid fermenting anaerobes: the Stickland reaction revisited. Can J Microbiol. 1982 Mar;28(3):291–300. doi: 10.1139/m82-043. [DOI] [PubMed] [Google Scholar]
- CHANEY A. L., MARBACH E. P. Modified reagents for determination of urea and ammonia. Clin Chem. 1962 Apr;8:130–132. [PubMed] [Google Scholar]
- Coleman G. S. Rumen ciliate protozoa. Adv Parasitol. 1980;18:121–173. doi: 10.1016/s0065-308x(08)60399-1. [DOI] [PubMed] [Google Scholar]
- Conway E. J. An absorption apparatus for the micro-determination of certain volatile substances: The determination of urea and ammonia in body fluids. Biochem J. 1933;27(2):430–434. [PMC free article] [PubMed] [Google Scholar]
- Conway E. J., Byrne A. An absorption apparatus for the micro-determination of certain volatile substances: The micro-determination of ammonia. Biochem J. 1933;27(2):419–429. [PMC free article] [PubMed] [Google Scholar]
- DEHORITY B. A., JOHNSON R. R., BENTLEY O. G., MOXON A. L. Studies on the metabolism of valine, proline, leucine and isoleucine by rumen microorganisms in vitro. Arch Biochem Biophys. 1958 Nov;78(1):15–27. doi: 10.1016/0003-9861(58)90310-2. [DOI] [PubMed] [Google Scholar]
- EL-SHAZLY K. Degradation of protein in the rumen of the sheep. II. The action of rumen micro-organisms on amino acids. Biochem J. 1952 Aug;51(5):647–653. doi: 10.1042/bj0510647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eadie J. M., Gill J. C. The effect of the absence of rumen ciliate protozoa on growing lambs fed on a roughage-concentrate diet. Br J Nutr. 1971 Sep;26(2):155–167. doi: 10.1079/bjn19710022. [DOI] [PubMed] [Google Scholar]
- Forsberg C. W., Lovelock L. K., Krumholz L., Buchanan-Smith J. G. Protease activities of rumen protozoa. Appl Environ Microbiol. 1984 Jan;47(1):101–110. doi: 10.1128/aem.47.1.101-110.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS D., ELSDEN S. R. The fermentation of L-threonine, L-serine, L-cysteine and acrylic acid by a gram-negative coccus. Biochem J. 1955 Aug;60(4):683–692. doi: 10.1042/bj0600683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Knight M. Concentrations of nicotinamide nucleotide coenzymes in micro-organisms. J Gen Microbiol. 1966 Aug;44(2):241–254. doi: 10.1099/00221287-44-2-241. [DOI] [PubMed] [Google Scholar]
- Massey L. K., Sokatch J. R., Conrad R. S. Branched-chain amino acid catabolism in bacteria. Bacteriol Rev. 1976 Mar;40(1):42–54. doi: 10.1128/br.40.1.42-54.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISMAN B., MAGER J. Diphosphopyridine nucleotide and phosphate requirement for oxidation of amino-acids by cell-free extracts of obligate anaerobes. Nature. 1952 Feb 9;169(4293):243–244. doi: 10.1038/169243a0. [DOI] [PubMed] [Google Scholar]
- NISMAN B. The Stickland reaction. Bacteriol Rev. 1954 Mar;18(1):16–42. doi: 10.1128/br.18.1.16-42.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLSON J. A., ANFINSEN C. B. Kinetic and equilibrium studies on crystalline 1-glutamic acid dehydrogenase. J Biol Chem. 1953 Jun;202(2):841–856. [PubMed] [Google Scholar]
- Palmquist D. L., Baldwin R. L. Enzymatic techniques for the study of pathways of carbohydrate utilization in the rumen. Appl Microbiol. 1966 Jan;14(1):60–69. doi: 10.1128/am.14.1.60-69.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B. Fermentation of Peptides by Bacteroides ruminicola B(1)4. Appl Environ Microbiol. 1983 May;45(5):1566–1574. doi: 10.1128/aem.45.5.1566-1574.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Jeraci J. L. Effect of carbon monoxide on fermentation of fiber, starch, and amino acids by mixed rumen microorganisms in vitro. Appl Environ Microbiol. 1984 Jul;48(1):211–217. doi: 10.1128/aem.48.1.211-217.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANWAL B. D., ZINK M. W. L-Leucine dehydrogenase of Bacillus cereus. Arch Biochem Biophys. 1961 Sep;94:430–435. doi: 10.1016/0003-9861(61)90070-4. [DOI] [PubMed] [Google Scholar]
- Satter L. D., Roffler R. E. Nitrogen requirement and utilization in dairy cattle. J Dairy Sci. 1975 Aug;58(8):1219–1237. doi: 10.3168/jds.S0022-0302(75)84698-4. [DOI] [PubMed] [Google Scholar]
- Stickland L. H. Studies in the metabolism of the strict anaerobes (genus Clostridium): The chemical reactions by which Cl. sporogenes obtains its energy. Biochem J. 1934;28(5):1746–1759. doi: 10.1042/bj0281746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Käufer B., Zähringer M., Jungermann K. The reaction of the iron-sulfur protein hydrogenase with carbon monoxide. Eur J Biochem. 1974 Mar 1;42(2):447–452. doi: 10.1111/j.1432-1033.1974.tb03358.x. [DOI] [PubMed] [Google Scholar]
- Tsubota H., Hoshino S. Transaminase activity in sheep rumen content. J Dairy Sci. 1969 Dec;52(12):2024–2028. doi: 10.3168/jds.S0022-0302(69)86890-6. [DOI] [PubMed] [Google Scholar]
- WALKER D. J. The purification and properties of the L-threonine deaminase of the rumen micro-organism LC1. Biochem J. 1958 Aug;69(4):524–530. doi: 10.1042/bj0690524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright D. E., Hungate R. E. Metabolism of glycine by rumen microorganisms. Appl Microbiol. 1967 Jan;15(1):152–157. doi: 10.1128/am.15.1.152-157.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y. Pyridine Nucleotide Content in the Higher Plant. Effect of Age of Tissue. Plant Physiol. 1963 Jan;38(1):45–54. doi: 10.1104/pp.38.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]


