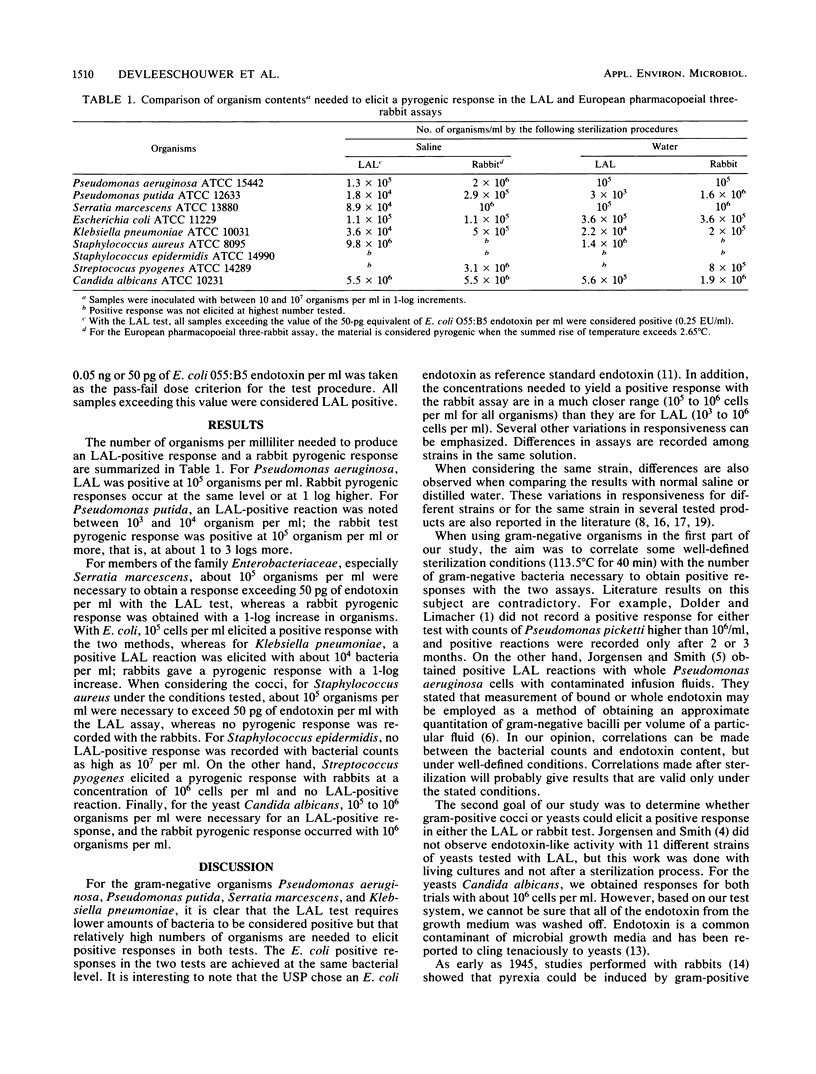
Abstract
The sensitivity and specificity of the Limulus amebocyte lysate test and rabbit pyrogen assay were studied by means of artificially contaminated parenterals. Various gram-negative and gram-positive bacterial strains were used as was one strain of the yeast Candida albicans. The numbers of organisms needed to elicit positive responses in distilled water and normal saline were recorded and compared. The sensitivity and specificity of the Limulus amebocyte lysate assay for the detection of bacterial endotoxin from gram-negative bacteria were demonstrated. Variable results were recorded with gram-positive bacteria and Candida albicans.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fine D. H., Kessler R. E., Tabak L. A., Shockman G. D. Limulus lysate activity of lipoteichoic acids. J Dent Res. 1977 Dec;56(12):1500–1500. doi: 10.1177/00220345770560121501. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. H., Smith R. F. Measurement of bound and free endotoxin by the Limulus assay. Proc Soc Exp Biol Med. 1974 Sep;146(4):1024–1031. doi: 10.3181/00379727-146-38240. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. H., Smith R. F. Preparation, sensitivity, and specificity of Limulus lysate for endotoxin assay. Appl Microbiol. 1973 Jul;26(1):43–48. doi: 10.1128/am.26.1.43-48.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J. H., Smith R. F. Rapid detection of contaminated intravenous fluids using the Limulus in vitro endotoxin assay. Appl Microbiol. 1973 Oct;26(4):521–524. doi: 10.1128/am.26.4.521-524.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Kinoshita F., Kato K., Harada K. Gelation of the amoebocyte lysate of Tachypleus tridentatus by cell wall digest of several gram-positive bacteria and synthetic peptidoglycan subunits of natural and unnatural configurations. Biken J. 1977 Mar;20(1):5–10. [PubMed] [Google Scholar]
- Mascoli C. C., Weary M. E. Limulus amebocyte lysate (LAL) test for detecting pyrogens in parenteral injectable products and medical devices: advantages to manufacturers and regulatory officials. J Parenter Drug Assoc. 1979 Mar-Apr;33(2):81–95. [PubMed] [Google Scholar]
- Nandan R., Brown D. R. An improved in vitro pyrogen test: to detect picograms of endotoxin contamination in intravenous fluids using limulus amoebocyte lysate. J Lab Clin Med. 1977 Apr;89(4):910–918. [PubMed] [Google Scholar]
- Pearson F. C., Dubczak J., Nakashima C., Carpenter D. F. Effect of nonsterility on the activity of Limulus amebocyte lysate. J Parenter Sci Technol. 1982 Sep-Oct;36(5):196–198. [PubMed] [Google Scholar]
- Pearson F. C. The Limulus amebocyte lysate endotoxin assay: current status. Am J Med Technol. 1979 Aug;45(8):704–709. [PubMed] [Google Scholar]
- Probey T. F., Pittman M. The Pyrogenicity of Bacterial Contaminants Found in Biologic Products. J Bacteriol. 1945 Oct;50(4):397–411. [PMC free article] [PubMed] [Google Scholar]
- Reinhold R. B., Fine J. A technique for quantitative measurement of endotoxin in human plasma. Proc Soc Exp Biol Med. 1971 May;137(1):334–340. doi: 10.3181/00379727-137-35572. [DOI] [PubMed] [Google Scholar]
- Ronneberger H. J. Comparison of the pyrogen tests in rabbits and with limulus lysate. Dev Biol Stand. 1977;34:27–36. [PubMed] [Google Scholar]
- Weary M. E., Donohue G., Pearson F. C., Story K. Relative potencies of four reference endotoxin standards as measured by the Limulus amoebocyte lysate and USP rabbit pyrogen tests. Appl Environ Microbiol. 1980 Dec;40(6):1148–1151. doi: 10.1128/aem.40.6.1148-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weary M., Baker B. Utilization of the limulus amebocyte lysate test for pyrogen testing large volume parenterals, administration sets, and medical devices. Bull Parenter Drug Assoc. 1977 May-Jun;31(3):127–133. [PubMed] [Google Scholar]
- Wildfeuer A., Heymer B., Schleifer K. H., Haferkamp O. Investigations on the specificity of the Limulus test for the detection of endotoxin. Appl Microbiol. 1974 Nov;28(5):867–871. doi: 10.1128/am.28.5.867-871.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildfeuer A., Heymer B., Schleifer K. H., Seidel H. P., Haferkamp O. Zur Schockdiagnostik: Der Nachweis von Endotoxin und Mucopeptid mit dem Limulus polyphemus-Lysat-Test. Klin Wochenschr. 1974 Feb 15;52(4):175–178. doi: 10.1007/BF01614393. [DOI] [PubMed] [Google Scholar]
- van Noordwijk J., de Jong Y. Comparison of the Limulus test for endotoxin with the rabbit test for pyrogens of European Pharmacopoeia. J Biol Stand. 1976 Apr;4(2):131–139. doi: 10.1016/0092-1157(76)90023-8. [DOI] [PubMed] [Google Scholar]


