Abstract
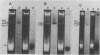
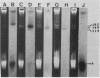
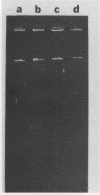
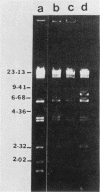
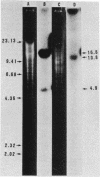
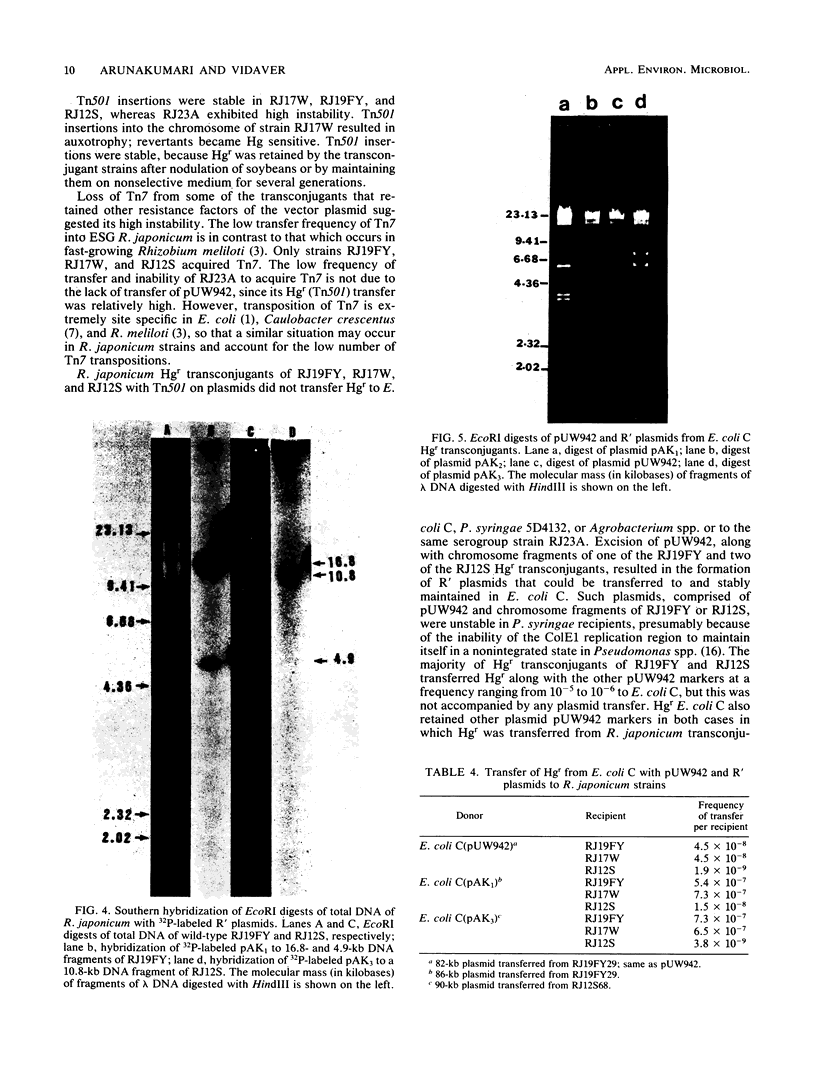
Transposons Tn501 (specifying mercury resistance) and Tn7 (specifying resistance to trimethoprim and streptomycin) were introduced into extra-slow-growing Rhizobium japonicum by conjugal transfer of the 82 kilobase chimeric plasmid pUW942. Mercury-resistant transconjugants were obtained at a frequency of 10−7 to 10−9. The transfer frequency of streptomycin resistance was lower than that of mercury resistance, and Tn7 was relatively unstable. pUW942 was not maintained as an autonomously replicating plasmid in R. japonicum strains. However, some of the Hgr transconjugants from the RJ19FY, RJ17W, and RJ12S strains acquired antibiotic markers of the vector plasmid pUW942. Southern hybridization of plasmid and chromosomal DNA of R. japonicum strains with 32P-labeled pUW942 and pAS8Rep-1, the same plasmid as pUW942 except that it does not contain Tn501, revealed the formation of cointegrates between pUW942 and the chromosome of R. japonicum. More transconjugants with only Tn501 insertions in plasmids or the chromosome were obtained in crosses with strains RJ19FY and RJ17W than with RJ12S. These retained stable Hgr both in plant nodules and under nonselective in vitro growth conditions. One of the RJ19FY and two of the RJ12S Hgr transconjugants with vector plasmid-chromosome cointegrates conjugally transferred plasmids of 82, 84 or 86, and 90 kilobases, respectively, into plasmidless Escherichia coli C. These plasmids strongly hybridized to pUW942 and EcoRI digests of total DNA of each respective R. japonicum strain but not to indigenous plasmid DNA of the R. japonicum strains. These R′ plasmids consisted of pUW942-specific EcoRI fragments and an additional one or two new fragments derived from the R. japonicum chromosome.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barth P. T., Datta N., Hedges R. W., Grinter N. J. Transposition of a deoxyribonucleic acid sequence encoding trimethoprim and streptomycin resistances from R483 to other replicons. J Bacteriol. 1976 Mar;125(3):800–810. doi: 10.1128/jb.125.3.800-810.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J. O., Atherly A. G. Induced plasmid-genome rearrangements in Rhizobium japonicum. J Bacteriol. 1984 Jan;157(1):218–224. doi: 10.1128/jb.157.1.218-224.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M. A., Elkan G. H. Transmissible resistance to penicillin G, neomycin, and chloramphenicol in Rhizobium japonicum. Antimicrob Agents Chemother. 1973 Sep;4(3):248–253. doi: 10.1128/aac.4.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean H. F., Morgan A. F. Integration of R91-5::Tn501 into the Pseudomonas putida PPN chromosome and genetic circularity of the chromosomal map. J Bacteriol. 1983 Jan;153(1):485–497. doi: 10.1128/jb.153.1.485-497.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely B. Transposition of Tn7 occurs at a single site on the Caulobacter crescentus chromosome. J Bacteriol. 1982 Aug;151(2):1056–1058. doi: 10.1128/jb.151.2.1056-1058.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrai T., Vincze E., Bánfalvi Z., Kiss G. B., Randhawa G. S., Kondorosi A. Localization of symbiotic mutations in Rhizobium meliloti. J Bacteriol. 1983 Feb;153(2):635–643. doi: 10.1128/jb.153.2.635-643.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg C., Casse-Delbart F., Dusha I., David M., Boucher C. Megaplasmids in the plant-associated bacteria Rhizobium meliloti and Pseudomonas solanacearum. J Bacteriol. 1982 Apr;150(1):402–406. doi: 10.1128/jb.150.1.402-406.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- Sato M., Staskawicz B. J., Panopoulos N. J., Peters S., Honma M. A host-dependent hybrid plasmid suitable as a suicidal carrier for transposable elements. Plasmid. 1981 Nov;6(3):325–331. doi: 10.1016/0147-619x(81)90040-8. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Vidaver A. K. Synthetic and complex media for the rapid detection of fluorescence of phytopathogenic pseudomonads: effect of the carbon source. Appl Microbiol. 1967 Nov;15(6):1523–1524. doi: 10.1128/am.15.6.1523-1524.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Falkow S. Transposon insertion and subsequent donor formation promoted by Tn501 in Bordetella pertussis. J Bacteriol. 1983 Jan;153(1):304–309. doi: 10.1128/jb.153.1.304-309.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]