Abstract
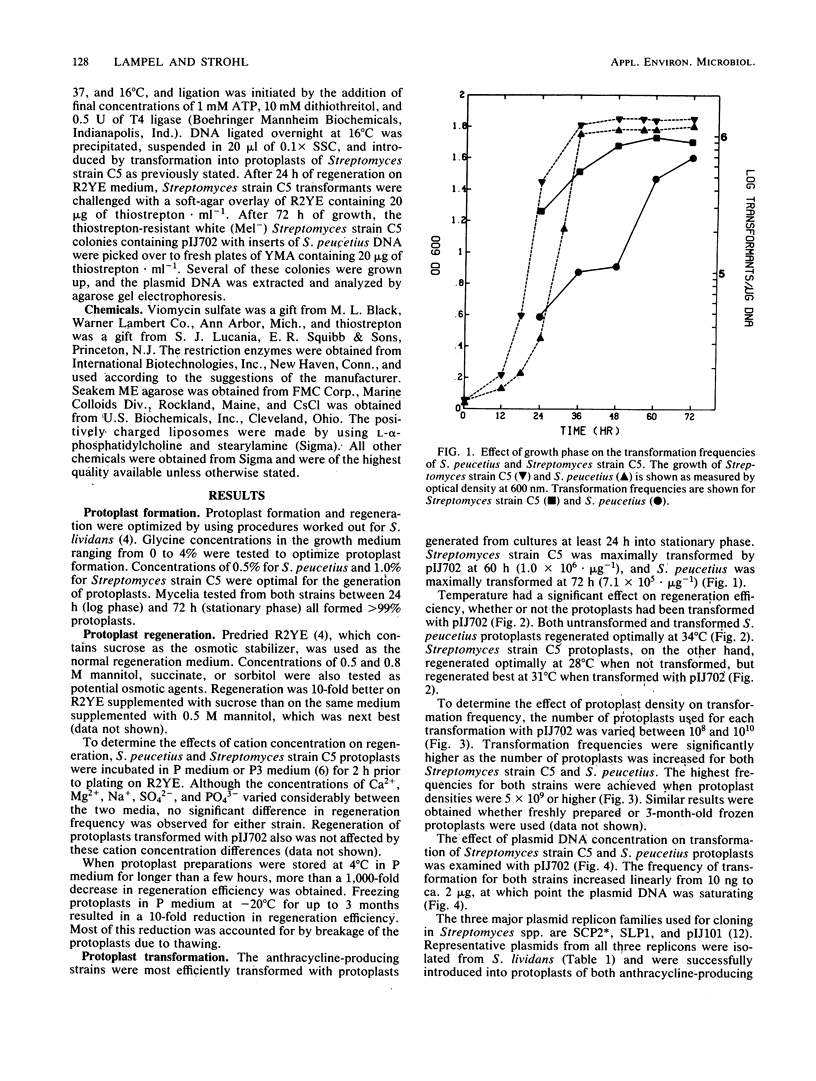
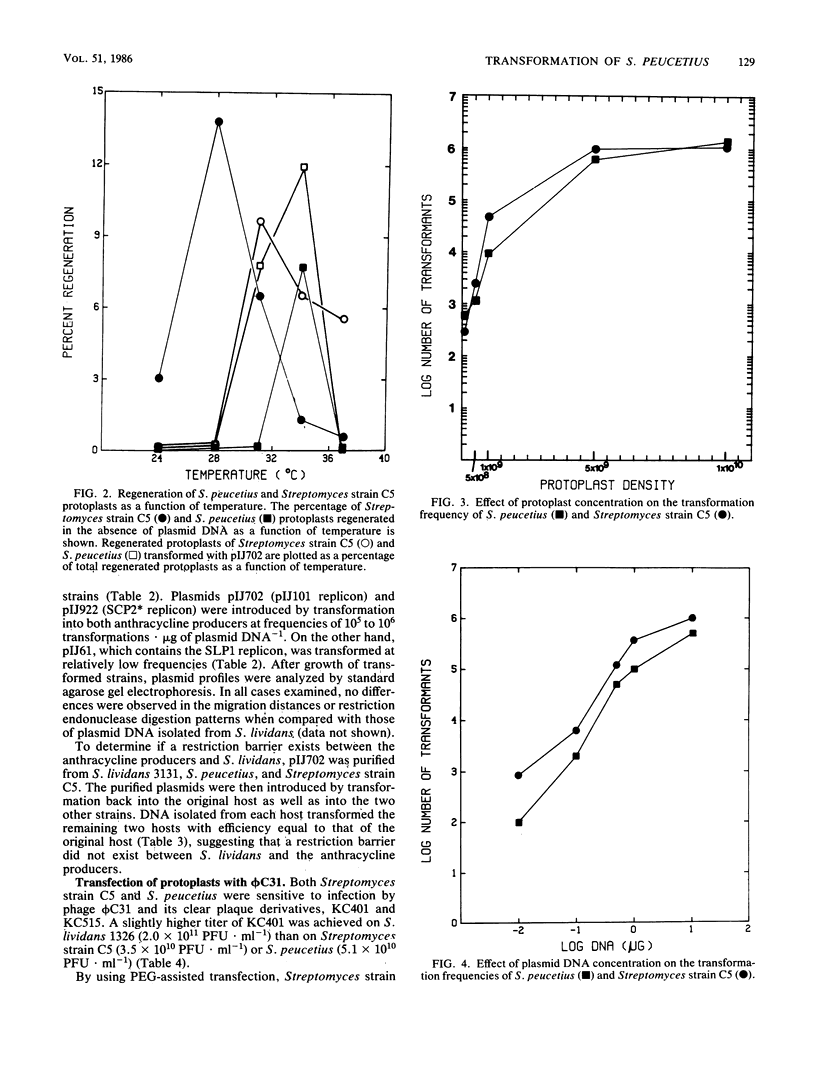
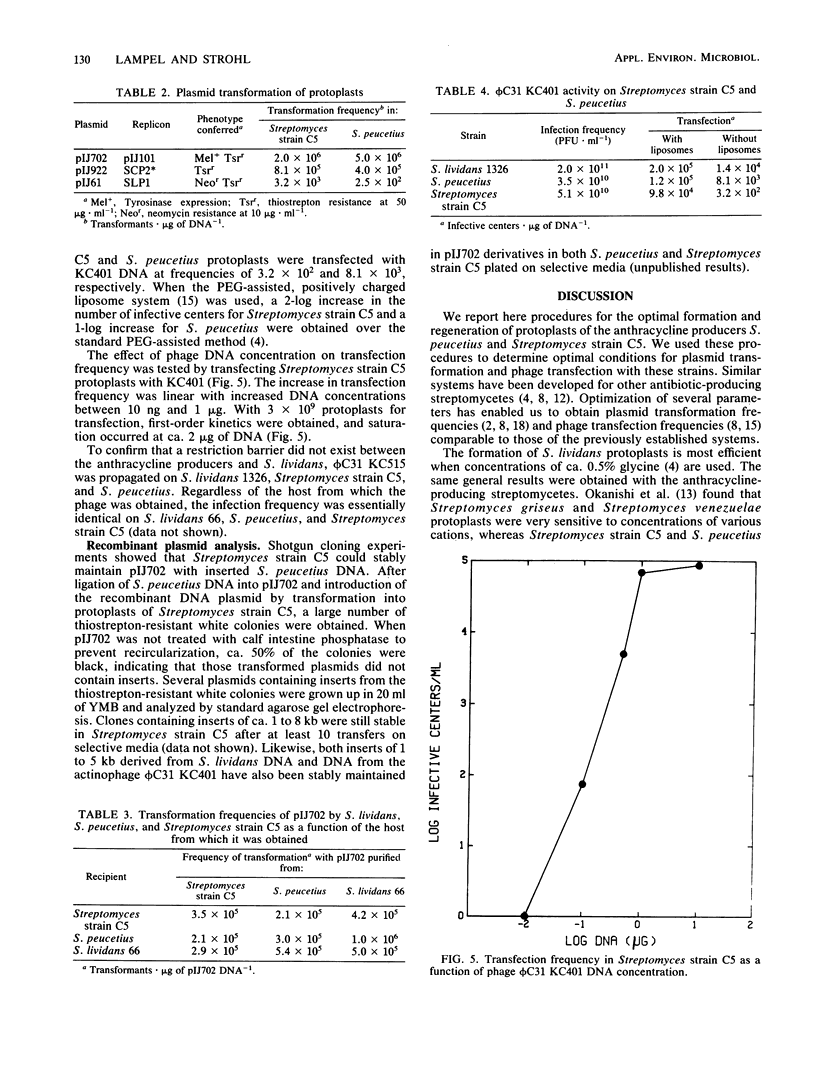
Streptomyces peucetius and Streptomyces strain C5, producers or anthracycline antibiotics, were converted to protoplasts from vegetatively growing mycelia. Conditions are described for maximal protoplast formation (greater than 99%) and for regeneration frequencies of up to 13%. Streptomycete plasmids pIJ61, pIJ702, and pIJ922, from the replicons SLP1, pIJ101, and SCP2, respectively, were isolated from Streptomyces lividans 66 and successfully introduced into S. peucetius and Streptomyces strain C5 by polyethylene glycol-mediated protoplast transformation. Frequencies of up to 10(6) transformations X microgram of plasmid DNA-1 were achieved by these procedures. Analyses showed that the two anthracycline-producing strains can stably harbor the plasmids without deletion of plasmid sequences or loss of the plasmids for several transfers through selective media. Fragments of DNA from S. peucetius ligated into pIJ702 and introduced into Streptomyces strain C5 were stable after several transfers through selective media. Both anthracycline producers also were sensitive to infection and transfection by actinophages KC401 and KC515, clear plaque derivatives of bacteriophage phi C31. Optimal conditions were determined for the transfection of S. peucetius and Streptomyces strain C5 protoplasts with phi C31 KC401 and KC515 DNA with liposome-assisted, polyethylene glycol-mediated protoplast transfection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltz R. H. Genetic recombination in Streptomyces fradiae by protoplast fusion and cell regeneration. J Gen Microbiol. 1978 Jul;107(1):93–102. doi: 10.1099/00221287-107-1-93. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Ward J. M., Hopwood D. A. Transformation of plasmid DNA into Streptomyces at high frequency. Nature. 1978 Jul 27;274(5669):398–400. doi: 10.1038/274398a0. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater K. F., Hopwood D. A., Kieser T., Thompson C. J. Gene cloning in Streptomyces. Curr Top Microbiol Immunol. 1982;96:69–95. doi: 10.1007/978-3-642-68315-2_5. [DOI] [PubMed] [Google Scholar]
- Chater K. F., Wilde L. C. Restriction of a bacteriophage of Streptomyces albus G involving endonuclease SalI. J Bacteriol. 1976 Nov;128(2):644–650. doi: 10.1128/jb.128.2.644-650.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekleva M. L., Titus J. A., Strohl W. R. Nutrient effects on anthracycline production by Streptomyces peucetius in a defined medium. Can J Microbiol. 1985 Mar;31(3):287–294. doi: 10.1139/m85-053. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Malpartida F., Kieser H. M., Ikeda H., Duncan J., Fujii I., Rudd B. A., Floss H. G., Omura S. Production of 'hybrid' antibiotics by genetic engineering. Nature. 1985 Apr 18;314(6012):642–644. doi: 10.1038/314642a0. [DOI] [PubMed] [Google Scholar]
- Katz E., Thompson C. J., Hopwood D. A. Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J Gen Microbiol. 1983 Sep;129(9):2703–2714. doi: 10.1099/00221287-129-9-2703. [DOI] [PubMed] [Google Scholar]
- Kieser T. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid. 1984 Jul;12(1):19–36. doi: 10.1016/0147-619x(84)90063-5. [DOI] [PubMed] [Google Scholar]
- Okanishi M., Suzuki K., Umezawa H. Formation and reversion of Streptomycete protoplasts: cultural condition and morphological study. J Gen Microbiol. 1974 Feb;80(2):389–400. doi: 10.1099/00221287-80-2-389. [DOI] [PubMed] [Google Scholar]
- Rodicio M. R., Chater K. F. Small DNA-free liposomes stimulate transfection of streptomyces protoplasts. J Bacteriol. 1982 Sep;151(3):1078–1085. doi: 10.1128/jb.151.3.1078-1085.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroshane R. M. Production of daunorubicin. Adv Biotechnol Processes. 1984;3:141–161. [PubMed] [Google Scholar]
- Suarez J. E., Chater K. F. Polyethylene glycol-assisted transfection of Streptomyces protoplasts. J Bacteriol. 1980 Apr;142(1):8–14. doi: 10.1128/jb.142.1.8-14.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. J., Ward J. M., Hopwood D. A. Cloning of antibiotic resistance and nutritional genes in streptomycetes. J Bacteriol. 1982 Aug;151(2):668–677. doi: 10.1128/jb.151.2.668-677.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voeikova T. A., Slavinskaia E. V., Orekhov A. V., Lomovskaia N. D. Identifikatsiia sistem restriktsii i modifikatsii u shtammov Streptomyces. Genetika. 1979;15(10):1746–1755. [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]


