Abstract
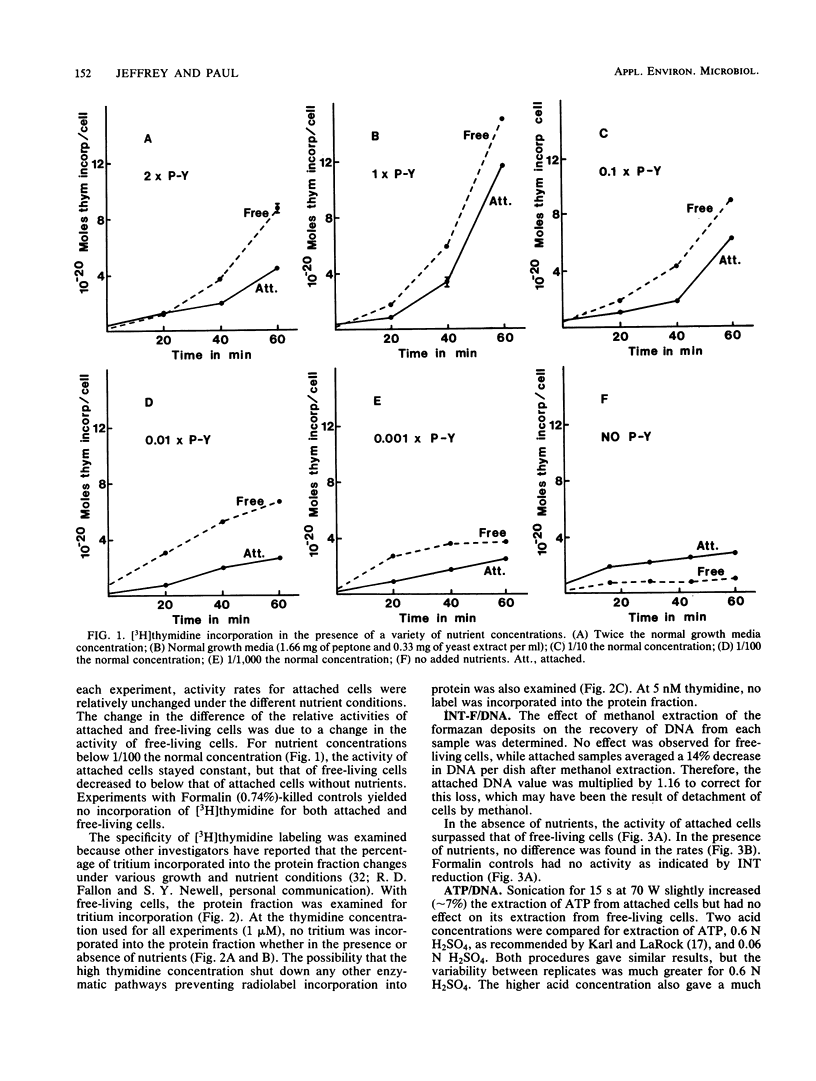
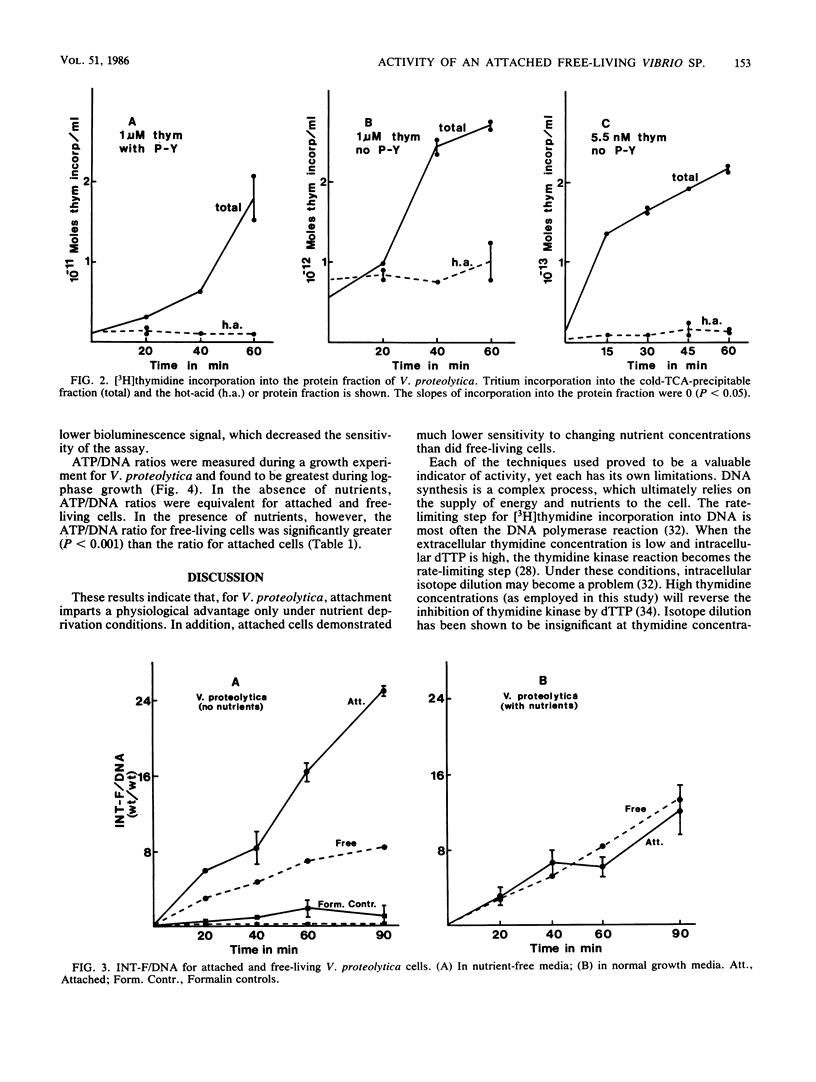
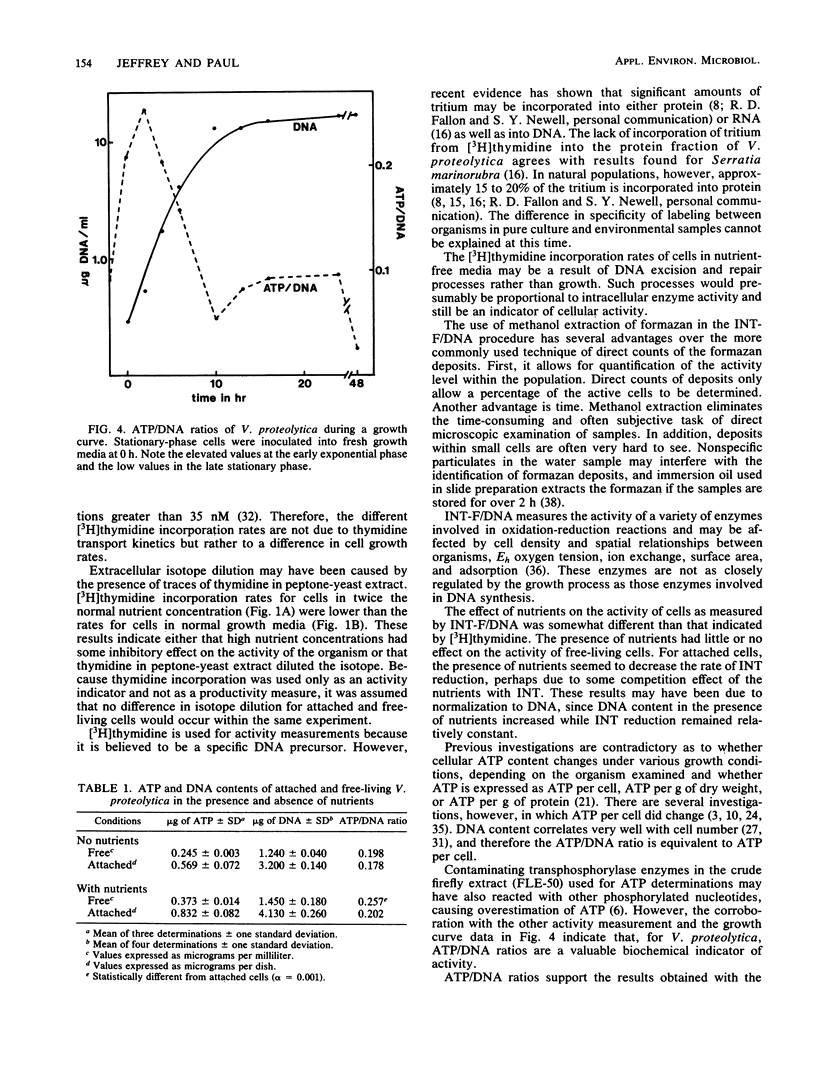
Three independent techniques, [3H]thymidine incorporation, the reduction rate of p-iodonitrotetrazolium violet (INT) to INT formazan normalized to DNA, and the ratio of ATP to DNA, were adapted to measure the activity of attached and unattached estuarine bacteria. In experiments employing the estuarine isolate Vibrio proteolytica, nutrient concentrations were manipulated by varying the concentration of peptone-yeast extract. In the presence of exogenous nutrients, the activity of free-living cells was greater than that of attached cells as measured by [3H]thymidine incorporation and ATP/DNA ratios. In the absence of peptone-yeast extract, however, the activity of attached cells surpassed that of free-living cells as determined by [3H]thymidine incorporation and INT formazan normalized to DNA. Of the three techniques, [3H]thymidine incorporation was deemed most sensitive for detecting changes in activity resulting from slight differences in nutrient concentration. By this technique, attached cells were much less sensitive to changing nutrient concentrations than were free-living cells. Below a threshold concentration, attached cell activity remained constant, while the activity of unattached cells decreased as a function of decreasing nutrient concentration. The results suggest that loss of cell surface area available for substrate uptake due to attachment may be an important factor in determining the relative activities of attached and free-living cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bright J. J., Fletcher M. Amino Acid assimilation and electron transport system activity in attached and free-living marine bacteria. Appl Environ Microbiol. 1983 Mar;45(3):818–825. doi: 10.1128/aem.45.3.818-825.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes E. A., Large P. J. Effect of starvation on the viability and cellular constituents of Zymomonas anaerobia and Zymomonas mobilis. J Gen Microbiol. 1970 Jan;60(1):31–42. doi: 10.1099/00221287-60-1-31. [DOI] [PubMed] [Google Scholar]
- Deluca M. Firefly luciferase. Adv Enzymol Relat Areas Mol Biol. 1976;44:37–68. doi: 10.1002/9780470122891.ch2. [DOI] [PubMed] [Google Scholar]
- Ellwood D. C., Keevil C. W., Marsh P. D., Brown C. M., Wardell J. N. Surface-associated growth. Philos Trans R Soc Lond B Biol Sci. 1982 Jun 11;297(1088):517–532. doi: 10.1098/rstb.1982.0058. [DOI] [PubMed] [Google Scholar]
- Gordon A. S., Gerchakov S. M., Millero F. J. Effects of inorganic particles on metabolism by a periphytic marine bacterium. Appl Environ Microbiol. 1983 Feb;45(2):411–417. doi: 10.1128/aem.45.2.411-417.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HATTORI T., FURUSAKA C. Chemical activities of Azotobacter agile adsorbed on a resin. J Biochem. 1961 Oct;50:312–315. doi: 10.1093/oxfordjournals.jbchem.a127450. [DOI] [PubMed] [Google Scholar]
- Jeffrey W. H., Paul J. H. Activity measurements of planktonic microbial and microfouling communities in a eutrophic estuary. Appl Environ Microbiol. 1986 Jan;51(1):157–162. doi: 10.1128/aem.51.1.157-162.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl D. M. Selected nucleic Acid precursors in studies of aquatic microbial ecology. Appl Environ Microbiol. 1982 Oct;44(4):891–902. doi: 10.1128/aem.44.4.891-902.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure K., Simidu U., Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979 Mar;25(3):415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- Lovell C. R., Konopka A. Thymidine incorporation by free-living and particle-bound bacteria in a eutrophic dimictic lake. Appl Environ Microbiol. 1985 Mar;49(3):501–504. doi: 10.1128/aem.49.3.501-504.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy W. F., Olson B. H. Fluorometric determination of the DNA concentration in municipal drinking water. Appl Environ Microbiol. 1985 Apr;49(4):811–817. doi: 10.1128/aem.49.4.811-817.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKAZAKI R., KORNBERG A. DEOXYTHYMIDINE KINASE OF ESCHERICHIA COLI. II. KINETICS AND FEEDBACK CONTROL. J Biol Chem. 1964 Jan;239:275–284. [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. The survival of starved bacteria. J Gen Microbiol. 1962 Oct;29:233–263. doi: 10.1099/00221287-29-2-233. [DOI] [PubMed] [Google Scholar]
- Paul J. H., Loeb G. I. Improved Microfouling Assay Employing a DNA-Specific Fluorochrome and Polystyrene as Substratum. Appl Environ Microbiol. 1983 Aug;46(2):338–343. doi: 10.1128/aem.46.2.338-343.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. H., Myers B. Fluorometric determination of DNA in aquatic microorganisms by use of hoechst 33258. Appl Environ Microbiol. 1982 Jun;43(6):1393–1399. doi: 10.1128/aem.43.6.1393-1399.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. H. Use of hoechst dyes 33258 and 33342 for enumeration of attached and planktonic bacteria. Appl Environ Microbiol. 1982 Apr;43(4):939–944. doi: 10.1128/aem.43.4.939-944.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard P. C., Moriarty D. J. Validity of the tritiated thymidine method for estimating bacterial growth rates: measurement of isotope dilution during DNA synthesis. Appl Environ Microbiol. 1984 Dec;48(6):1076–1083. doi: 10.1128/aem.48.6.1076-1083.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH R. C., MAALOE O. EFFECT OF GROWTH RATE ON THE ACID-SOLUBLE NUCLEOTIDE COMPOSITION OF SALMONELLA TYPHIMURIUM. Biochim Biophys Acta. 1964 May 11;86:229–234. doi: 10.1016/0304-4165(64)90047-9. [DOI] [PubMed] [Google Scholar]
- Sjostrom D. A., Forsdyke D. R. Isotope-dilution analysis of rate-limiting steps and pools affecting the incorporation of thymidine and deoxycytidine into cultured thymus cells. Biochem J. 1974 Feb;138(2):253–262. doi: 10.1042/bj1380253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevors J. T. Electron transport system activity in soil, sediment, and pure cultures. Crit Rev Microbiol. 1984;11(2):83–100. doi: 10.3109/10408418409105473. [DOI] [PubMed] [Google Scholar]
- Zimmermann R., Iturriaga R., Becker-Birck J. Simultaneous determination of the total number of aquatic bacteria and the number thereof involved in respiration. Appl Environ Microbiol. 1978 Dec;36(6):926–935. doi: 10.1128/aem.36.6.926-935.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobell C. E. The Effect of Solid Surfaces upon Bacterial Activity. J Bacteriol. 1943 Jul;46(1):39–56. doi: 10.1128/jb.46.1.39-56.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]


