Abstract
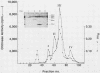
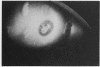
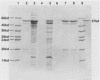
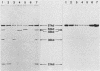
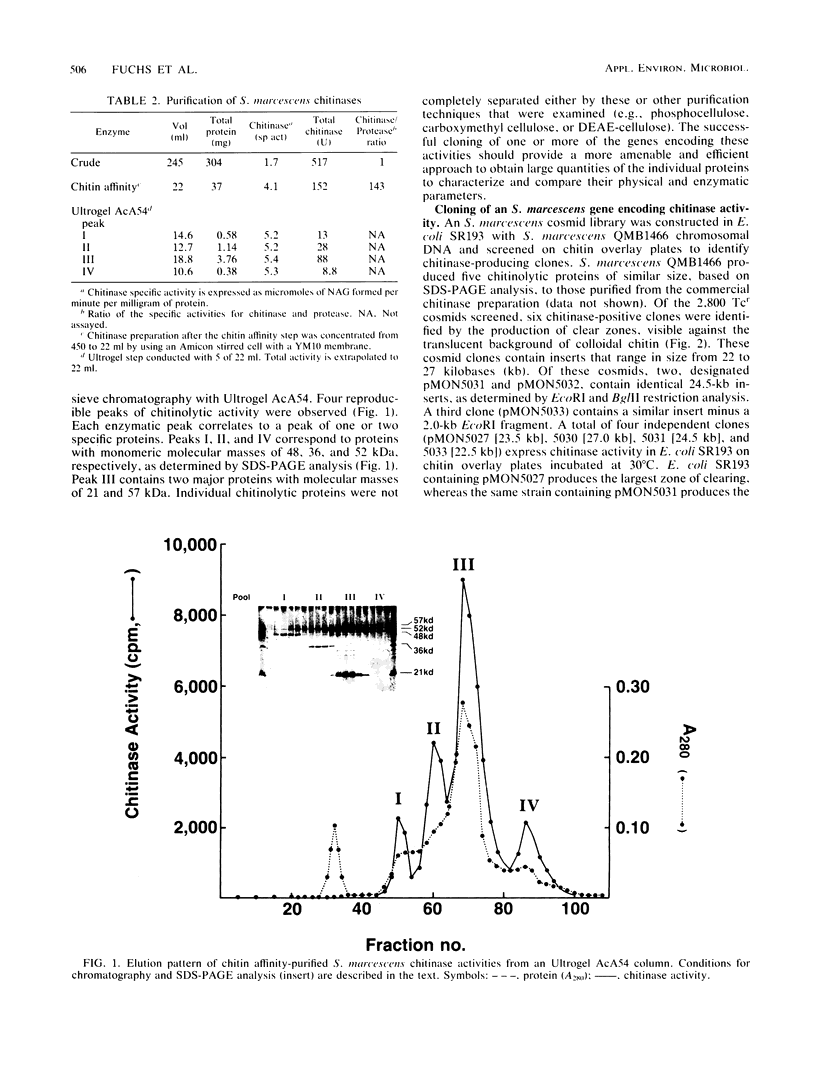
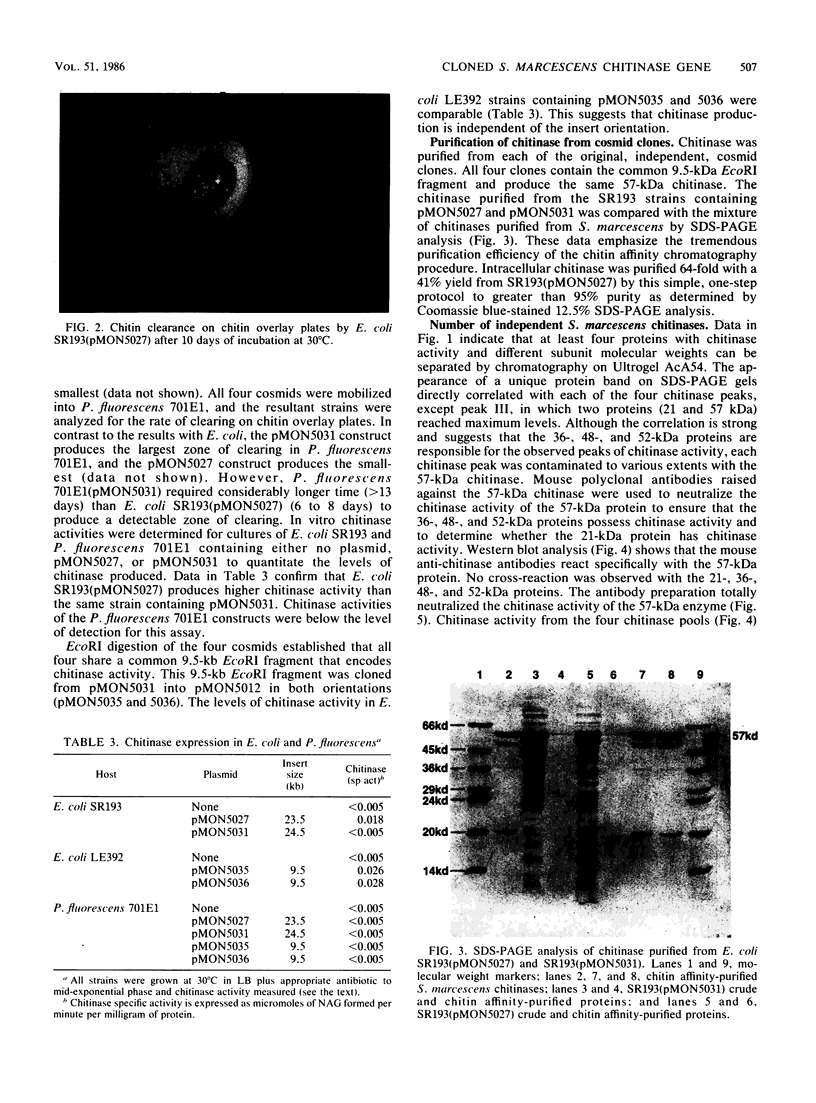
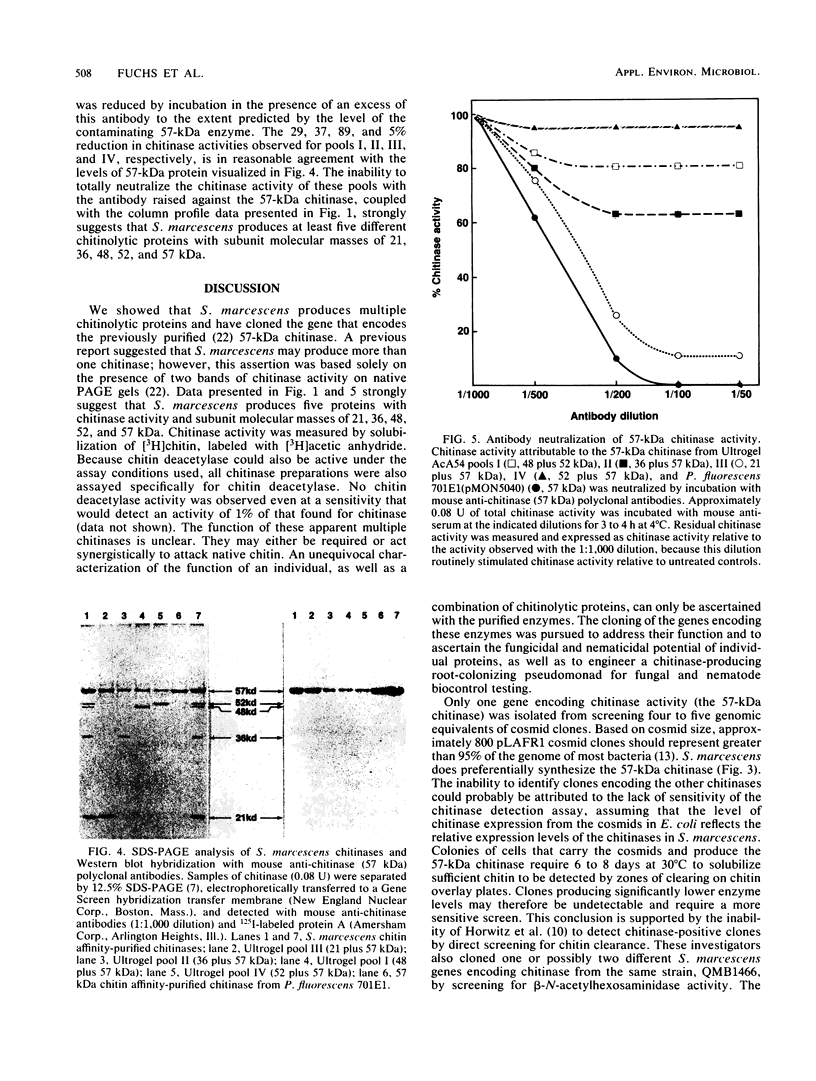
Serratia marcescens, a chitinase-producing microorganism, was shown to produce five unique chitinolytic proteins with subunit molecular masses of 21, 36, 48, 52, and 57 kilodaltons. A cosmid library of S. marcescens DNA was constructed in the broad-host-range cosmid pLAFR1 and screened in Escherichia coli for clones capable of degrading chitin. A total of four independent clones (22- to 27-kilobase inserts) were isolated, characterized by restriction endonuclease digestion, and shown to share a common 9.5-kilobase EcoR1 fragment apparently encoding the same 57-kilodalton chitinase, the most abundant chitinase produced by S. marcescens. Chitinase expression from these constructs in both E. coli and Pseudomonas fluorescens 701E1 is apparently driven by an S. marcescens promoter. The significantly higher chitinase levels produced in E. coli relative to those in P. fluorescens 701E1 suggest that E. coli may recognize this promoter sequence more efficiently than P. fluorescens.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki Y., Ito E. A pathway of chitosan formation in Mucor rouxii: enzymatic deacetylation of chitin. Biochem Biophys Res Commun. 1974 Feb 4;56(3):669–675. doi: 10.1016/0006-291x(74)90657-3. [DOI] [PubMed] [Google Scholar]
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R. L., Kane J. F. In vivo synthesis of histidine by a cloned histidine ammonia-lyase in Escherichia coli. J Bacteriol. 1985 Apr;162(1):98–101. doi: 10.1128/jb.162.1.98-101.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Long S., Mothibeli M. A., Robb F. T., Woods D. R. Regulation of extracellular alkaline protease activity by histidine in a collagenolytic Vibrio alginolyticus strain. J Gen Microbiol. 1981 Nov;127(1):193–199. doi: 10.1099/00221287-127-1-193. [DOI] [PubMed] [Google Scholar]
- Molano J., Durán A., Cabib E. A rapid and sensitive assay for chitinase using tritiated chitin. Anal Biochem. 1977 Dec;83(2):648–656. doi: 10.1016/0003-2697(77)90069-0. [DOI] [PubMed] [Google Scholar]
- Monreal J., Reese E. T. The chitinase of Serratia marcescens. Can J Microbiol. 1969 Jul;15(7):689–696. doi: 10.1139/m69-122. [DOI] [PubMed] [Google Scholar]
- Roberts R. L., Cabib E. Serratia marcescens chitinase: one-step purification and use for the determination of chitin. Anal Biochem. 1982 Dec;127(2):402–412. doi: 10.1016/0003-2697(82)90194-4. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]