Abstract
Two methods are commonly used to measure the community metabolism (primary production, respiration, and calcification) of shallow-water marine communities and infer air–sea CO2 fluxes: the pH-total alkalinity and pH-O2 techniques. The underlying assumptions of each technique are examined to assess the recent claim that the most widely used technique in coral reefs (pH-total alkalinity), may have provided spurious results in the past because of high rates of nitrification and release of phosphoric acid in the water column [Chisholm, J. R. M. & Barnes, D. J. (1998) Proc. Natl. Acad. Sci. USA 95, 6566–6569]. At least three lines of evidence suggest that this claim is not founded. First, the rate of nitrification required to explain the discrepancy between the two methods recently reported is not realistic as it is much higher than the rates measured in another reef system and greater than the highest rate measured in a marine environment. Second, fluxes of ammonium, nitrate, and phosphorus are not consistent with high rates of nitrification and release of phosphoric acid. Third, the consistency of the metabolic parameters obtained by using the two techniques is in good agreement in two sites recently investigated. The pH-total alkalinity technique therefore appears to be applicable in most coral reef systems. Consequently, the conclusion that most coral reef flats are sources of CO2 to the atmosphere does not need revision. Furthermore, we provide geochemical evidence that calcification in coral reefs, as well as in other calcifying ecosystems, is a long-term source of CO2 for the atmosphere.
The contribution of an ecosystem to the global carbon cycle primarily results from (i) the balance between organic carbon production (photosynthetic CO2 fixation) and consumption (respiratory CO2 release) and (ii) the balance between calcium carbonate precipitation (a source of CO2) and dissolution (a sink for CO2) (1). Two methods are used to measure community metabolism of calcifying communities in flowing seawater: the alkalinity anomaly technique [pH-TA (2, 3)] and the pH-O2 technique (4). In a recent paper, Chisholm and Barnes (5) cast doubt on the validity of the former method and suggest that it may explain the recent controversy on the role of coral reefs in the global carbon cycle. The validity of techniques used to estimate the metabolism of coastal marine communities is a critical issue at a time where unprecedented international programs (Land-Ocean Interaction in the Coastal Zone, LOICZ, and European Land-Ocean Interaction Studies, ELOISE) seek to estimate the contribution of the coastal zone to the global carbon cycle.
The aim of the present paper is to examine the assumptions involved in the pH-TA and pH-O2 techniques, to assess the claims of Chisholm and Barnes (5) by using both published and unpublished data, and to address the problem of the effect of reef metabolism on the global carbon cycle, in both the short and the long term.
The pH-TA Technique.
This technique is based on measurements of pH and total alkalinity (TA) upstream and downstream of a community. These variables are used to calculate the difference between the downstream and upstream concentrations of dissolved inorganic carbon (ΔDIC) by using standard equations that describe the seawater inorganic carbon system (e.g., ref. 6). The rate of calcification is calculated by using the simple stoichiometric relationship that relates ΔTA and community net calcification:
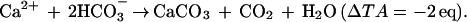 |
1 |
The 1:1 molar relationship between the CO2 released and the CaCO3 precipitated holds in freshwater only, and the ratio is lower than 1 in seawater because of its buffering capacity (7). Calcification is therefore a CO2-releasing process that can make water in equilibrium with the atmosphere degas, against the initial pCO2 gradient (8). Total alkalinity and DIC decrease by 2 eq and 1 mol, respectively, for each mole of CaCO3 precipitated. This change of DIC resulting from calcification (ΔDICcalc) is then subtracted from ΔDIC to provide an estimate of net community production (ΔDICorg). Further details on the pH-TA technique are given by Smith and Key (2) and Smith and Kinsey (3). The major assumption of this method is that no process other than calcification significantly affects TA during the transit of the water mass above the community investigated. Removal of CO2 by photosynthesis and its addition by respiration have no effect on TA. However, these processes are coupled with the assimilation and dissimilation of NH4+, NO3− and HPO42− which liberate OH− or H+ (or uptake H+ or OH−):
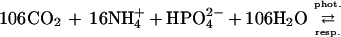 |
2a |
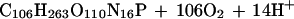 |
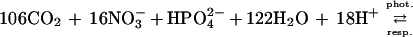 |
2b |
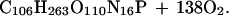 |
The forward reactions induce ΔTA of, respectively, −0.13 and +0.17 equivalents per mole of fixed CO2. Eqs. 2a and 2b are based on the Redfield C:N:P atomic ratio that was derived for marine plankton (9). Distinct ratios were found in other communities leading to a stoichiometry of photosynthesis and respiration different from those shown here. The stoichiometry found in benthic marine plants (10) results in a ΔTA of +0.06 eq per mole of fixed CO2.
Nitrification (Eq. 3) and sulfate reduction (Eq. 4) also significantly alter TA:
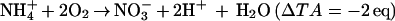 |
3 |
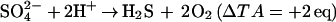 |
4 |
Kinsey (11) has demonstrated that processes 2–4 are likely to cause errors of less than 5% on the net community calcification in a number of reefs. There certainly are environments, such as sedimentary lagoons, in which reaction 4 can be significant (11). Organic acids can significantly contribute to total alkalinity in eutrophic environments (12), but their concentration is likely to be very low in most reef settings. Additionally, organic acid alkalinity behaves nearly conservatively in organic rich estuaries of Georgia (12). Extending these observations to the open ocean and to coral reefs, which have both similar DOC concentrations but much lower levels of organic acids than estuaries, seems reasonable. Errors in the absolute total alkalinity should thus be minor and relatively constant. Because community metabolism deals with changes in TA, not absolute values, the effects are negligible. Finally, the validity of the alkalinity anomaly technique to estimate coral calcification has been repeatedly established (3, 13, 14).
The pH-O2 Technique.
This technique was first introduced by Barnes (4) to circumvent the major drawback of the pH-TA method. There is no simple way to monitor TA remotely, by probe, as the water mass flows over the community; it is therefore required that discrete seawater samples be taken for subsequent measurements of TA in the laboratory to calculate ΔTA. The pH-O2 technique uses relationships between ΔO2 and ΔDICorg, the metabolic quotients¶, to estimate net community production and respiration from changes in the concentration of dissolved oxygen. ΔDICcalc is then calculated by subtracting ΔDICorg from the upstream DIC value, and ΔTA and net community calcification are estimated by using the stoichiometric relationships described above (Eq. 1). This approach is attractive because precise and reliable sensors are available to monitor both dissolved oxygen and pH in the field, and application of the technique requires fewer determinations of TA (to estimate upstream DIC) than the pH-TA technique. However, this technique also requires the use of assumed values of the metabolic quotients, thus introducing uncertainty into the calculated metabolic parameters. Any biogeochemical process that has an O2:CO2 stoichiometry different from the assumed metabolic quotients interferes with the pH-O2 technique. In particular, note that this is the case with nitrification (Eq. 5), which consumes 2 mol of O2 per mol of NH4+ nitrified. The pH-O2 technique is therefore affected by nitrification just as much as the pH-TA technique.
Estimation of Air–Sea CO2 Fluxes.
The contribution of ecosystems to exchange of CO2 between ocean and atmosphere can be estimated by using direct measurements (15, 16) or using parameters of community metabolism (1). A simple expression was derived to estimate the amount of DIC (FCO2) that needs to be exchanged with the atmosphere to restore the carbonate equilibrium (1):
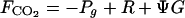 |
5a |
or
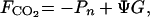 |
5b |
where the community gross primary production (Pg), net primary production (or net ecosystem production, Pn), respiration (R), and net calcification (G) are expressed in molar units, and Ψ is the molar ratio of CO2 released by calcification to calcium carbonate precipitated. Positive FCO2 indicate CO2 evasion (sea to air flux) and negative FCO2 indicate CO2 invasion (air to sea flux). Frankignoulle et al. (7) have shown that Ψ = 0.6 for seawater displaying the following characteristics: pCO2 = 356 μatm, TA = 2370 μeq kg−1, temperature = 25°C and salinity = 35 (1 atm = 101.3 kPa).
This relationship enables us to compute values of the G/Pn ratio that are important to identify the effect of coral reefs on seawater pCO2 (Table 1). The critical ratio that causes no change in pCO2 (and no air–sea CO2 flux) is G/Pn = 1.67. The system becomes a source of CO2 when G/Pn > 1.67 and a sink for CO2 when G/Pn < 1.67. Most reef flats exhibit G/Pn ratios higher than 1.67/1.0 because their metabolism of organic carbon is nearly balanced (Pg/R ≈ 1, Pn ≈ 0) (13). It has therefore been suggested that most reef flats can be expected to cause CO2 evasion to the atmosphere (1), a prediction that has been confirmed by a limited number of direct measurements (15, 16).
Table 1.
Effect of community metabolism on the seawater carbonate system and air–sea CO2 flux
| G/Pn, mol/mol | ΔTA/ΔDIC, equivalent/mol | FCO2, mol |
|---|---|---|
| 1.0/0.0 (net calcification only) | −2.0/−1.0 | +0.6 ↗ |
| 1.67/1.0 | −3.33/−2.67 | 0.0 → |
| 1.0/1.0 | −2.0 /−2.0 | −0.4 ↘ |
| 0.0/1.0 (net photosynthesis only) | 0.0 /−1.0 | −1.0 ↘ |
G, net community calcification; Pn, net community primary production, ΔTA, change in total alkalinity; ΔDIC, change in the concentration of dissolved inorganic carbon; FCO2, amount of DIC that needs to be exchanged with the atmosphere in order to restore the carbonate equilibrium. The arrows indicate the direction of the air–sea CO2 flux: ↗, evasion; →, no flux; ↘, invasion. The small changes in total alkalinity resulting from assimilation and excretion of nutrients associated with production and degradation of organic carbon were neglected.
Discrepancy Between the pH-TA and pH-O2 Techniques Reported by Chisholm and Barnes.
These authors (5) investigated the community metabolism of a fringing reef flat at Lizard Island (Great Barrier Reef, Australia). Salinity ranged from 1 to 3 units below normal values because of heavy rainfall before the measurements. They estimated the rate of community calcification (G) by using both the pH-TA and the pH-O2 techniques. During daylight, G estimated from pH-O2 data was similar to that estimated from pH-TA data (41.0 and 36.8 g CaCO3 m−2). However, the two methods did not compare well at night: there was net CaCO3 dissolution according to the pH-O2 data (G = −15.5 g CaCO3 m−2) and net CaCO3 deposition according the pH-TA technique (G = 4.7 g CaCO3 m−2).
Consequently, the pH-TA technique provided estimates of annual rates of net calcification higher than the pH-O2 technique (ca. 15.1 vs. 9.3 kg CaCO3 m−2 yr−1, respectively). The former rate was deemed unrealistically high for a reef exhibiting only 0–10% of live coral cover.
Chisholm and Barnes (5) infer that substantial decomposition of organic matter, due to the low salinity at the time of the experiments, explains the high rate of calcification estimated with the pH-TA technique. They then generalize this suggestion and conclude that “(D)ata presented herein question the long standing assumption that the carbonate equilibrium of seawater above most reefs is principally controlled by photosynthesis, respiration, calcification and solution of reef rock.” Chisholm and Barnes (5) invoke two processes that may have affected the total alkalinity during their experiments: the formation of phosphoric acid due to organic matter decomposition (Eqs. 2a and 2b) and a “considerably greater amount of nitric acid via oxidation of ammonium by nitrifying bacteria” (Eq. 3).
We will evaluate this claim by using three approaches: (i) assessing whether the rate of nitrification required to explain the discrepancy between the two methods at Lizard Island is realistic, (ii) examining whether high rates of organic matter decomposition and nitrification are likely in two other sites, and (iii) checking the consistencies of measured ΔTA and ΔTA estimated by the pH-O2 technique, as well as metabolic quotients, at these sites.
Estimation of the Nitrification Rate Required to Explain the Discrepancy Reported by Chisholm and Barnes.
The decomposition of organic matter releases NH4+ (2a) that can be subsequently nitrified (2b and 3). It either increases TA by 0.13 eq per mol of carbon respired (2a) or decreases TA by 0.17 eq per mol of carbon respired (2b). Nitrification (3) decreases TA by 2 eq per mol of ammonium nitrified. None of these processes was measured by Chisholm and Barnes (5), but one can estimate the rates required to explain the nighttime difference in calcification between the ΔTA and pH-O2 techniques (−47 + 155 = 108 mmol CaCO3 m−2). Because the difference was attributed to calcification, the change in TA is two times higher than the change in CaCO3. Therefore, the two processes invoked by Chisholm and Barnes (5) must have decreased TA by 216 meq m−2 or, assuming a 12:12 h photoperiod, 18 meq m−2 h−1. They estimate that the effect of nitrification was considerably higher than that of organic matter decomposition. Let us assume that those processes accounted for, respectively, 90% and 10% of the excess change in TA. Therefore, the rate of nighttime nitrification must have been around 8 mmol m−2 h−1 (18 × 0.9/2). Nitrification is known to occur in corals (17) and coral reefs (18). However, the rates reported are much lower than 8 mmol m−2 h−1. Webb and Wiebe (18) reported rates ranging from 0.4 to 39 μmol m−2 h−1 (mean = 10.4 μmol m−2 h−1; N = 6) at Enewetak. Two reviews compile rates of nitrification measured in marine sediment (19) and in coastal marine environments (20). The highest rate was measured in North Sea sediments and reaches 1.4 mmol m−2 h−1 (21). Also, coral reef waters lack the substrate required for intense nitrification because ammonium concentration typically ranges between 0.2 and 0.5 μmol liter−1 (22). Ammonium enrichment experiments carried out on sediment of the Lizard Island reef flat have shown that a significant ammonium uptake is obtained when its concentration in the overlying water is 5 mmol liter−1 (J. W. Bishop, personal communication). Note that the nighttime uptake rate [2.35 ± 0.44 (±SD) mmol m−2 h−1] is still three times lower than that supposed to have occurred at the same site. It is therefore concluded that the nitrification rate required to support the Chisholm and Barnes hypothesis is very unlikely to occur as it is much higher than the rates measured in a coral reef and other marine environments by 1 to 3 orders of magnitude.
Do High Rates of Nitrification and Release of Phosphoric Acid Occur at Other Sites?
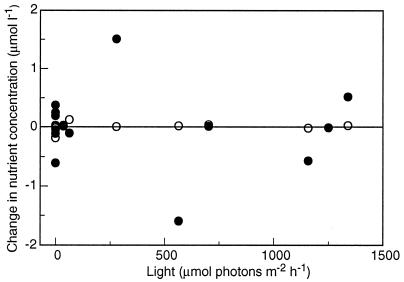
These processes should induce significant changes in the nutrient concentration of the water mass as it flows across the reef flat. However, as mentioned by Gattuso et al. (23): “The contribution of nutrients to changes in total alkalinity was negligible at Yonge reef since there was no or very small changes in nutrient concentrations during the transects (data not shown).” The data are shown in Fig. 1. The changes in NH4+ and PO43− during the transect experiments were not statistically significant different from 0 (P = 0.09–0.97), whether at night or during the day. The average areal fluxes are −0.019 mmol PO43− m−2 h−1 and 0.083 mmol NH4+ m−2 h−1. We therefore conclude that no significant nitrification took place at the time of measurement. It is likely that a similar situation prevailed at Moorea. Recent observations demonstrate that no nitrification takes place in a reef located in southern Japan (24): NH4+ does not change significantly, there is no release of NO3− at any time, and a significant NO3− uptake was measured at night (as opposed to NO3− release if nitrification occurred).
Figure 1.
Changes in the concentrations of phosphorus (squares) and ammonium (circles) during transects carried out at Yonge Reef (J.-P.G., M. Pichon, B. Delesalle, C. Canon, and M. Frankignoulle, unpublished results). Dissolved inorganic nutrients were measured on a Skalar multichannel segmented flow autoanalyzer (57). The analytical method is based on that of Tréguer and Le Corre (58) modified to ensure a suitable accuracy and sensitivity to low concentration levels, as well as the linearity of the response over a wide range of concentrations.
Comparison of the pH-TA and pH-O2 Data at Other Sites.
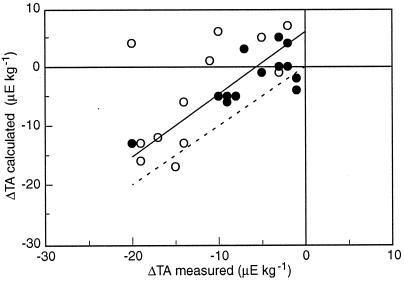
The relationship between measured ΔTA and ΔTA estimated by the pH-O2 method can be investigated by using the data sets collected on two Pacific barrier‖ reef flats at Moorea and Yonge Reef by Gattuso et al. (15, 23). The two methods are well correlated (r = 0.68; P < 0.001; Fig. 2). The slope of the geometric regression line is not significantly different from 1 and the y intercept (6 μeq kg−1) is significantly different from 0. Several limitations of the pH-O2 method could explain such slight discrepancy. First, pH and DIC are known to provide poor estimates of TA (e.g., ref. 26) and the accuracy of calculated ΔTA depends on the accuracy of both pH and O2. If one sets the error of the latter variables to very small values (0.003 unit for pH and 3 μmol kg−1 for O2), the resulting error on ΔTA is 7 μeq kg−1. Such an error is within the ranges of measured ΔTA (see Fig. 2). Second, the estimate of ΔTA is very sensitive to the correction because of air–sea O2 exchange (FO2). The largest difference between ΔTA measured and ΔTA estimated by the pH-O2 method shown in Fig. 2 (24 μeq kg−1) could result from an inaccuracy of FO2 of only 20%. Chisholm and Barnes (5) estimated FO2 from a relationship between the gas exchange coefficient and wind speed. This procedure does not properly account the effect of inner turbulence due to stresses other than wind (current and bottom topography). Frankignoulle et al. (16) demonstrated that the CO2 gas exchange coefficient, which exhibits a similar response to wind speed and inner turbulence than the O2 gas exchange coefficient, is underestimated by more than 50% when turbulence is not taken into account.
Figure 2.
Change in total alkalinity estimated by the pH-O2 technique as a function of measured change in total alkalinity. Data at Moorea (open circles) and Yonge Reef (filled circles) are from Gattuso et al. (23). The 1:1 and the geometric regression lines (y = 6 + 1.06 x; N = 27; r = 0.68) are shown as dotted and plain lines, respectively.
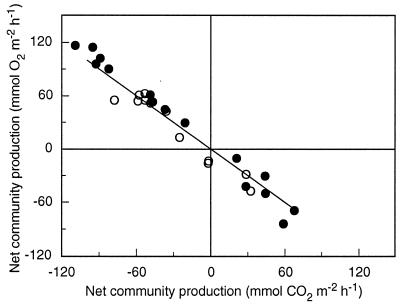
The rates of net community production obtained by the pH-O2 and pH-TA techniques should not be consistent if high rates of nitrification and release of phosphoric acid occur, and anomalous values of metabolic quotients should be found. That did not happen at Moorea (1992) or at Yonge Reef (Fig. 3). Net community production rates estimated with both techniques were consistent and highly correlated (r2 was 0.93 and 0.98, respectively, at Moorea and Yonge Reef). The slopes were not statistically different from 1. The data set collected at Moorea in 1991 cannot be analyzed in a similar way because the CO2 data were not reliable, because of a relatively poor accuracy of the pH measurements (15).
Figure 3.
Net community production estimated using the O2 and CO2 techniques at Yonge Reef (filled circles) and Moorea (open circles) (23). The 1:1 line is shown.
The average respiratory quotient (RQ) was 0.9 ± 0.2 at Moorea (N = 2) and 0.8 ± 0.1 at Yonge Reef (N = 3). It was significantly lower than 1 (P = 0.04) at Yonge Reef. PQnet was 1 ± 0.1 at Moorea (N = 5) and 1.1 ± 0.1 at Yonge Reef (N = 10); they were not significantly different than 1 (P > 0.25). True PQ was not significantly different at Moorea (1.07 ± 0.06; N = 10) and Yonge Reef (1.08 ± 0.03; N = 12). Therefore, the metabolic quotients measured at these two sites are perfectly normal and agree with those with those obtained by various authors at other sites (e.g., ref. 27). The nutrient data, the comparison of measured and estimated TA, as well as the consistency of the metabolic quotients demonstrate that none of the processes invoked by Chisholm and Barnes (5) distorted the parameters of community metabolism measured at Moorea and Yonge Reef.
Contribution of Coral Reefs to the Carbon Cycle.
After questioning the reliability of the results obtained using the pH-TA technique, Chisholm and Barnes (5) implied that the distortion of the estimates of community production and calcification, mostly due to nitrification, observed at Lizard Island may “explain apparent anomalies in the metabolic performance of reefs close to land and reconcile the different experimental findings that have given rise to the CO2 debate.” The so-called “CO2 debate” relates (i) to a discrepancy between short-term measurements and estimates of air–sea CO2 fluxes over reef flats by two of the authors (J.-P. G. and M. F.), and some estimates measured by other authors and (ii) to the long-term role of coral reefs on the global carbon cycle.
A large number of reports demonstrate that coral reef ecosystems, mostly barrier reef flats, are sources of carbon dioxide to the atmosphere because of their low net fixation of CO2 via photosynthetic processes (net community production is close to 0) and rather large release of CO2 by precipitation of calcium carbonate (1, 7, 8, 15, 16, 23, 28–30). The reef investigated at Moorea by Gattuso et al. (15) did not “apparently release(d) CO2 to the atmosphere” as is suggested by Chisholm and Barnes (5). Air–sea CO2 evasion was not only inferred by using an indirect method based on community metabolism data but was also directly measured. Similar measurements carried out at Moorea (in a different season) and Yonge Reef provided the same result (16). The reversal of the measured air–sea CO2 flux that occurs during late morning and early night takes place when the seawater pCO2 computed from pH and TA is approximately 355 μatm, the value of atmospheric pCO2 at the time of measurement (16). This demonstrates that estimation of seawater pCO2 from pH and TA is satisfactory. There was a 1 to 2 orders of magnitude difference in the daily air–sea CO2 fluxes estimated from direct measurements and from parameters of the community metabolism (16, 23). These authors have provided several considerations that could explain such a difference. The point, however, is that they both indicate a CO2 efflux to the atmosphere. Also, Chisholm and Barnes (5) did not realize that these two approaches used the same TA data.
Some recent reports suggest, however, that some reef flats are sinks for atmospheric CO2 (31–34). Some of the later conclusions are, however, hampered by the techniques used, the limited data sets, and the representativity of the study sites (35) and are not consistent with reef sediment geochemistry (36). It is significant that most studies suggesting that reefs may be sinks of CO2 were carried out on fringing reefs, which are more likely subject to anthropogenic stresses than other reef systems. There is an increasing number of reefs shifting from coral-dominated to algal-dominated states (e.g., refs. 37–39) because of factors related, to some extent, to human-induced changes. The effect of these changes on the ecosystem function are poorly known, but it has often been suggested that they lead to an increase in the community excess production and a decrease in community calcification (36, 40, 41). These responses may shift the ecosystem from a CO2 source for the atmosphere to a CO2 sink (42, 43). Critics of the Shiraho (Ryukyu Island, Japan) reef study by Kayanne et al. (31) did not “argue … that the reef must have been dominated by noncalcareous algae … ” as stated by Chisholm and Barnes (5). They based their argument on qualitative surveys carried out in December 1994 (M. Pichon, personal communication) and October 1995 (J.-P.G., unpublished observations) which showed that the Shiraho reef flat exhibits large areas with 100% algal cover and that the sedimentary area located along the shore harbors seagrass beds (42).
Therefore, the conclusion that “average” coral reef flats are sources of CO2 to the atmosphere (1) still stands. Whereas average coral reef flats behaves as sources of CO2 for the atmosphere, reefs are essentially balanced ecosystems. Compared with reef flat studies, integrated studies encompass a much larger area, include considerably more sedimentary zones (hence a much lower community net calcification) and integrate the CO2-related signals over many days. It was shown that the CO2 flux associated with calcification and the CO2 flux associated with organic metabolism almost exactly offset in Spencer Gulf (not a coral reef but nevertheless a calcifying system; ref. 44). Similar investigations in atolls reached a similar conclusion (e.g., ref. 45). Calcification makes the system degas CO2. Organic metabolism makes the CO2 flux go either in or out, depending on trophic status. Because most reefs seem to be marginally autotrophic, the tendency of organic metabolism will be one of slight CO2 invasion. Our point is that there is a tendency of the two processes of organic production and calcification to at least compensate one another in complete reef systems while CO2 invasion due to organic metabolism is overwhelmed by the CO2 released by calcification in most reef flats.
Chisholm and Barnes (5) also state that “(reefs) may release up to 8% (of anthropogenic CO2) if they are sources” and quote Gattuso et al. (23) to support that statement. The latter paper does not provide such estimate and, to our knowledge, the only estimate of CO2 release by reefs available in the literature is that of Ware et al. (8); 0.4–1% of anthropogenic CO2 release). Chisholm and Barnes (5) argue that “The fact that carbonate rocks stores 3 104 more inorganic carbon than the atmosphere (Skirrow, 1978) shows that reefs are sinks for CO2 over geological time.” This is a misunderstanding of simple geochemical reactions known since the last century (e.g., ref. 46). Such misconception has been published on several occasions in the 1990s, the first account being, perhaps, that of Karube et al. (47), who even proposed that calcification was a possible mechanism for absorbing some of the anthropogenic CO2 emissions. This misconception recognizes that the massive CaCO3 deposits associated with past and present reefs are sinks for carbon and makes the erroneous conclusion that they are sinks for atmospheric CO2. The oceans contain approximately 50 times more inorganic carbon than the atmosphere. There are three pools of oceanic DIC: HCO3− (90%), CO32− (9%), and dissolved CO2 (1%). The latter pool is close to equilibrium with the atmosphere (present pCO2 ca. 360 μatm). The carbon atom incorporated into CaCO3 is derived from the HCO3− pool, with the consequence that H+ is liberated and the water gets more acid. The acid pushes an additional amount of HCO3− across into the oceanic CO2 pool. There is then a physical equilibration between the seawater and atmosphere CO2 pools, and this physical equilibration pushes CO2 into the atmosphere.
Berner et al. (48) provided a very thorough account of the geochemical control of the changes of atmospheric carbon dioxide over the past 100 million years. Gases released by volcanoes, including H2S, NH3, CH4, and H2O, made up much of the highly reducing early atmosphere of earth. Reducing molecules, such as methane, became oxidized to form an early CO2-rich atmosphere. The concentration of CO2 was subsequently controlled by the carbonate-silicate geochemical cycle. The weathering of Ca-Mg silicate rocks followed by precipitation of Ca and Mg in carbonate minerals in the ocean is a major process by which CO2 is stored. These reactions are described by the Högbom-Urey reactions (49). For example, in the case of calcium silicate:
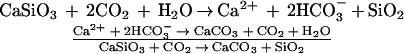 |
Additionally, according to Berner et al. (48), precipitation of CaCO3 is an important process and the major way by which CO2 is returned to the atmosphere. They also demonstrated that a 10% drop in the rate of addition of CO2 to the atmosphere via oceanic CaCO3 precipitation (all the other fluxes remaining constant) would result in the complete removal of atmospheric CO2 in only 30,000 years. There is therefore no doubt that reefs, as well as other calcifying systems, were sources of CO2 over geological time. Some authors believe that changes in coral reef calcification resulting from variations of sea level and climate are partly responsible for the 80 μatm difference in atmospheric pCO2 (200 vs. 280 μatm) during the last glacial–interglacial period (e.g., ref. 50), although that hypothesis (so-called coral reef hypothesis) was recently challenged (51).
Conclusions and Perspectives.
The data set collected at Lizard Island by Chisholm and Barnes (5) is puzzling and, so far, unique in the literature. It is, however, very unlikely that the anomalous rates of community metabolism that they reported solely result from high rates of organic matter decomposition and nitrification driven by extreme meteorological conditions prior to the measurements. We conclude that:
- 1.
The high rates of nitrification and release of phosphoric acid that are presumed to have distorted community metabolism data at Lizard Island (5) have not occurred in several sites previously investigated. Therefore, the pH-TA method remains widely applicable in most reef systems.
- 2.
The so-called controversy on the role of reef flats as source or sink of CO2 does not result from a methodological problem related to anomalous changes in total alkalinity at Moorea and Yonge Reef. In our opinion, the results suggesting that two fringing reefs of southern Japan are sinks for atmospheric CO2 are mostly due to a high surface cover of macrophytes and a low surface cover of corals. A recent paper provides evidence for this (42).
- 3.
Coral reefs are not a quantitatively important component of the present day global carbon cycle as their gross metabolic performance is about a 1–2% term in the marine biotic gross CO2 pump (28). Nevertheless, they played, together with other calcifying organisms and ecosystems, a quantitatively important role in the long-term control of atmospheric pCO2 by releasing significant amounts of CO2 (e.g., refs. 48 and 50).
It is our hope that this paper will clarify the assumptions and drawbacks of the methods commonly used to measure the community metabolism of marine communities. Also, the carbon and carbonate cycles of coral reefs are of great interest but the issue of their role in the global carbon cycle is now quite firmly established. There remain, however, many exciting questions of major interest such as the interactions between the two geochemical cycles (28, 52) as well as the consequences of global climatic changes on reef primary production (52) and calcification (37, 52–56).
Acknowledgments
Thanks are due to Analytical Services, Australian Institute of Marine Science, for performing the nutrient determinations reported in Fig. 1, to J. W. Bishop for providing results from a paper in preparation, to M. Pichon who provided unpublished information on the Shiraho reef system, and to R. van Woesik for making available a paper in press. M. F. is a Fonds National de la Recherche Scientifique (Belgium) research associate.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Community net photosynthetic quotient (PQnet = ΔO2/ΔDICorg) and community respiratory quotient (RQ = ΔDICorg/ΔO2).
It is worth emphasizing that the site investigated at Moorea (as well as at Yonge Reef) is a barrier reef and not a fringing reef as claimed by Chisholm and Barnes (5). The Tiahura reef system is much closer to the coast than Yonge reef and is subject to a relatively intense human pressure (25). However, the barrier reef is not greatly affected by the land because (i) it is hydrodynamically isolated from it by a channel exhibiting a very strong current and (ii) the water mass impinging on the reef crest only contains ca. 10% of water recirculated through the nearby pass (Wolanski et al., ref. 25).
References
- 1.Gattuso J-P, Pichon M, Frankignoulle M. Mar Ecol Prog Ser. 1995;129:307–312. [Google Scholar]
- 2.Smith S V, Key G S. Limnol Oceanogr. 1975;20:493–495. [Google Scholar]
- 3.Smith S V, Kinsey D W. In: Coral Reefs: Research Methods. Stoddart D R, Johannes R E, editors. Paris: Unesco; 1978. pp. 469–484. [Google Scholar]
- 4.Barnes D J. J Exp Mar Biol Ecol. 1983;66:149–161. [Google Scholar]
- 5.Chisholm J R M, Barnes D J. Proc Natl Acad Sci USA. 1998;95:6566–6569. doi: 10.1073/pnas.95.11.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skirrow G. In: Chemical Oceanography. 2nd Ed. Riley J P, Skirrow G, editors. Vol. 2. London: Academic Press; 1975. pp. 1–192. [Google Scholar]
- 7.Frankignoulle M, Canon C, Gattuso J-P. Limnol Oceanogr. 1994;39:458–462. [Google Scholar]
- 8.Ware J R, Smith S V, Reaka-Kudla M L. Coral Reefs. 1992;11:127–130. [Google Scholar]
- 9.Redfield A C, Ketchum B H, Richards F A. In: The sea. Hill M N, editor. Vol. 2. New York: Wiley; 1963. pp. 26–77. [Google Scholar]
- 10.Atkinson M J, Smith S V. Limnol Oceanogr. 1983;28:568–574. [Google Scholar]
- 11.Kinsey D W. Limnol Oceanogr. 1978;23:989–991. [Google Scholar]
- 12.Cai W J, Wang Y. Limnol Oceanogr. 1998;43:657–668. [Google Scholar]
- 13.Tambutté É, Allemand D, Bourge I, Gattuso J-P, Jaubert J. Mar Biol. 1995;122:453–459. [Google Scholar]
- 14.Chisholm J R M, Gattuso J-P. Limnol Oceanogr. 1991;36:1232–1239. [Google Scholar]
- 15.Gattuso J-P, Pichon M, Delesalle B, Frankignoulle M. Mar Ecol Prog Ser. 1993;96:259–267. [Google Scholar]
- 16.Frankignoulle M, Gattuso J-P, Biondo R, Bourge I, Copin-Montégut G, Pichon M. Mar Ecol Prog Ser. 1996;145:123–132. [Google Scholar]
- 17.Wafar M, Wafar S, David J J. Limnol Oceanogr. 1990;35:725–730. [Google Scholar]
- 18.Webb K L, Wiebe W J. Can J Microbiol. 1975;21:1427–1431. doi: 10.1139/m75-214. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan W A. In: Nitrogen in the Marine Environment. Carpenter E J, Capone D G, editors. New York: Academic; 1983. pp. 139–190. [Google Scholar]
- 20.Henriksen K, Kemp M. In: Nitrogen Cycling in Coastal Marine Environments. Blackburn T H, Sørensen J, editors. Chichester: Wiley; 1988. pp. 207–249. [Google Scholar]
- 21.Vanderborght J-P, Billen G. Limnol Oceanogr. 1975;20:953–961. [Google Scholar]
- 22.Crossland C J. In: Perspectives on Coral Reefs. Barnes D J, editor. Townsville: Australian Institute of Marine Science; 1983. pp. 56–68. [Google Scholar]
- 23.Gattuso J-P, Pichon M, Delesalle B, Canon C, Frankignoulle M. Mar Ecol Prog Ser. 1996;145:109–121. [Google Scholar]
- 24.Ohde S, van Woesik R. Bull Mar Sci. 1999;65:559–576. [Google Scholar]
- 25.Wolanski E, Delesalle B, Dufour V, Aubanel A. 11th Australasian Conference on Coastal and Ocean Engineering. Barton, Australia: Institution of Engineers; 1993. pp. 583–589. [Google Scholar]
- 26.Dickson A G, Riley J P. Mar Chem. 1978;6:77–85. [Google Scholar]
- 27.Kinsey D W. Ph.D. thesis. University of Hawaii; 1979. p. 248. [Google Scholar]
- 28.Smith S V. Kansas Geological Survey Open-File Report 95-96a. Lawrence, KS: Kansas Geological Survey; 1995. pp. 1–18. [Google Scholar]
- 29.Kawahata H, Suzuki A, Goto K. Coral Reefs. 1997;16:261–266. [Google Scholar]
- 30.Suzuki A, Kawahata H, Goto K. Proceedings 8th International Coral Reef Symposium. Balboa, Panamá, Panamá: Smithsonian Tropical Research Institute; 1997. pp. 971–976. [Google Scholar]
- 31.Kayanne H, Suzuki A, Saito H. Science. 1995;269:214–216. doi: 10.1126/science.269.5221.214. [DOI] [PubMed] [Google Scholar]
- 32.Yamamuro M, Kayanne H, Minagawa M. Limnol Oceanogr. 1995;40:617–621. [Google Scholar]
- 33.Kraines S, Suzuki Y, Omori T, Shitashima K, Kanahara S, Komiyama H. Mar Ecol Prog Ser. 1997;156:1–16. [Google Scholar]
- 34.Ikeda Y, Hata H, Suzuki A, Kayanne H. Proc. 8th Int. Coral Reef Symp. Balboa, Republic of Panamá: Smithsonian Tropical Research Institute; 1997. pp. 965–970. [Google Scholar]
- 35.Gattuso J-P, Frankignoulle M, Smith S V, Ware J R, Wollast R. Science. 1996;271:1298. doi: 10.1126/science.271.5253.1298a. [DOI] [PubMed] [Google Scholar]
- 36.Buddemeier R W. Science. 1996;271:1298–1299. doi: 10.1126/science.271.5253.1298b. [DOI] [PubMed] [Google Scholar]
- 37.Smith S V, Buddemeier R W. Annu Rev Ecol Syst. 1992;23:89–118. [Google Scholar]
- 38.Hughes T P. Science. 1994;265:1547–1551. doi: 10.1126/science.265.5178.1547. [DOI] [PubMed] [Google Scholar]
- 39.McClanahan T R, Muthiga N A. Environmental Conservation. 1998;25:122–130. [Google Scholar]
- 40.Kinsey D W. Galaxea. 1988;7:113–128. [Google Scholar]
- 41.Buddemeier R W. Kansas Geological Survey Open-File Report 95–96b. Lawrence, KS: Kansas Geological Survey; 1995. pp. 1–12. [Google Scholar]
- 42.Gattuso J-P, Payri C E, Pichon M, Delesalle B, Frankignoulle M. J Phycol. 1997;33:729–738. [Google Scholar]
- 43.Gattuso J-P, Frankignoulle M. International Workshop on CO2 Cycling and Metabolism in Coral Reefs. and New Energy and Industrial Technology Development Organization, Kyoto: Research Institute of Innovative Technology for the Earth; 1998. p. 8. [Google Scholar]
- 44.Smith S V, Veeh H H. Est Coast Shelf Sci. 1989;29:195–215. [Google Scholar]
- 45.Smith S V, Jokiel P L. In: An Environmental Survey of Canton Atoll Lagoon. Smith S V, Henderson R S, editors. San Diego: Naval Undersea Center; 1976. pp. 15–53. [Google Scholar]
- 46.Chamberlin T C. J Geol. 1898;6:609–621. [Google Scholar]
- 47.Karube I, Takeuchi T, Barnes D J. Adv Biochem Eng/Biotechnol. 1992;46:63–79. [Google Scholar]
- 48.Berner R A, Lasaga A C, Garrels R M. Am J Sci. 1983;283:641–683. [Google Scholar]
- 49.Berner R A. Am J Sci. 1995;295:491–495. [Google Scholar]
- 50.Opdyke B N, Walker J C G. Geology. 1992;20:733–736. doi: 10.1130/0091-7613(1992)020<0733:rotcrh>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 51.Broecker W S, Henderson G M. Paleoceanography. 1998;13:352–364. [Google Scholar]
- 52.Gattuso J-P, Allemand D, Frankignoulle M. Am Zool. 1999;39:160–183. [Google Scholar]
- 53.Gattuso J-P, Frankignoulle M, Bourge I, Romaine S, Buddemeier R W. Global Planet Change. 1998;18:37–46. [Google Scholar]
- 54.Buddemeier R W, Fautin D G. Bull Inst Océanogr, Monaco n° spéc. 1996;14:33–38. [Google Scholar]
- 55.Buddemeier R W, Fautin D G. Bull Inst Océanogr, Monaco n° spéc. 1996;14:23–32. [Google Scholar]
- 56.Kleypas J A, Buddemeier R W, Archer D, Gattuso J-P, Langdon C, Opdyke B N. Science. 1999;284:118–120. doi: 10.1126/science.284.5411.118. [DOI] [PubMed] [Google Scholar]
- 57.Ryle V D, Mueller H R, Gentien P. Aust Inst Mar Sci Tech Bull Oceanogr Ser. 1981;3:1–24. [Google Scholar]
- 58.Tréguer P, Le Corre P. Manuel d’analyse des sels nutritifs dans l’eau de mer (utilisation de l’autoanalyseur II Technicon) (Université de Bretagne Occidentale, Brest, France) 1975. [Google Scholar]