Abstract
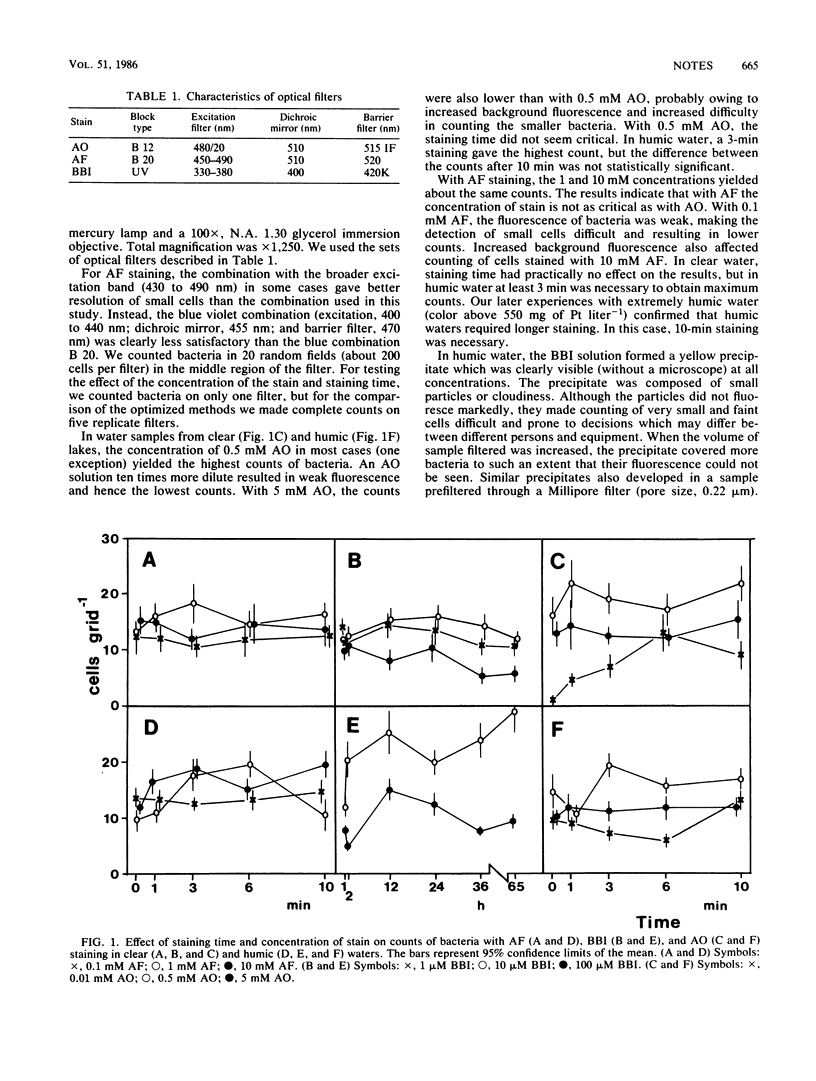
In highly humic water, acridine orange precipitated with dissolved humic matter, resulting in such bright background fluorescence that no bacteria could be seen. With bisbenzimide staining, a similar precipitate was nonfluorescent but obscured many cells. An acriflavine staining method proved useful and reproducible both in clear and in humic waters. Fading of fluorescence was not a problem, and stained samples could be stored after preparation. The fluorescence of cells stained with acriflavine was weaker than that with acridine orange, making counting extremely small cells slightly more difficult with the former stain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crissman H. A., Mullaney P. F., Steinkamp J. A. Methods and applications of flow systems for analysis and sorting of mammalian cells. Methods Cell Biol. 1975;9(0):179–246. doi: 10.1016/s0091-679x(08)60076-x. [DOI] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. G., Simon B. M. An investigation of errors in direct counts of aquatic bacteria by epifluorescence microscopy, with reference to a new method for dyeing membrane filters. J Appl Bacteriol. 1975 Dec;39(3):317–329. doi: 10.1111/j.1365-2672.1975.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Paul J. H. Use of hoechst dyes 33258 and 33342 for enumeration of attached and planktonic bacteria. Appl Environ Microbiol. 1982 Apr;43(4):939–944. doi: 10.1128/aem.43.4.939-944.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


