Abstract
Mucor fragilis grown on bovine blood powder as the sole carbon source abundantly produced β-N-acetylhexosaminidase. The enzyme activity was several times higher than that of a culture obtained with glucose medium. The enzyme had two different molecular weight forms. The high-molecular-weight form had somewhat higher β-N-acetylgalactosaminidase activity than the lower-molecular-weight enzyme which had β-N-acetylgalactosaminidase activity equivalent to about 40% of its β-N-acetylglucosaminidase activity. Bovine blood seemed to induce both enzymes, but N-acetylamino sugars specifically induced the low-molecular-weight form. N-Acetylgalactosamine had an especially marked effect on activity. The low-molecular-weight form of enzyme was purified from the culture filtrate by fractionation with ammonium sulfate and various column chromatographies. The purified enzyme was found to be homogeneous by polyacrylamide gel electrophoresis. The optimum pH was 4.0 to 5.0 for β-N-acetylglucosaminidase activity and 5.5 to 6.5 for β-N-acetylgalactosaminidase activity. The enzyme hydrolyzed natural substrates such as di-N-acetylchitobiose, tri-N-acetylchitotriose, and a glycopeptide obtained by modification of fetuin.
Full text
PDF
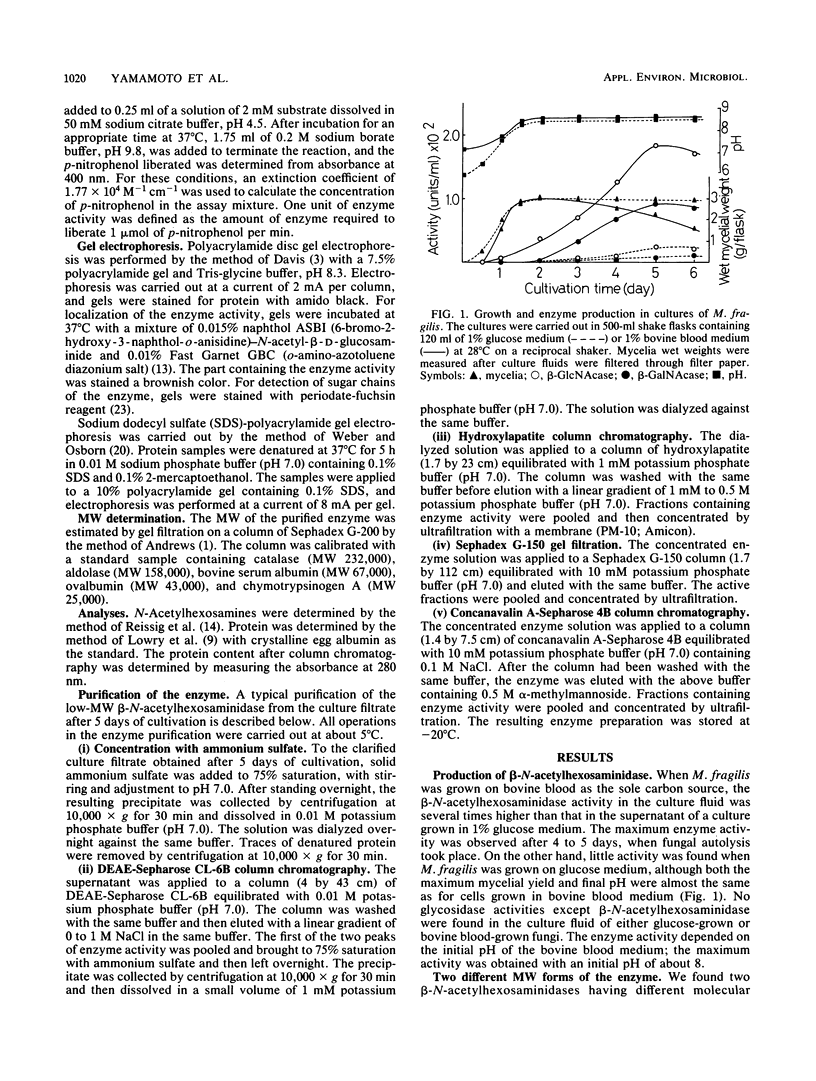
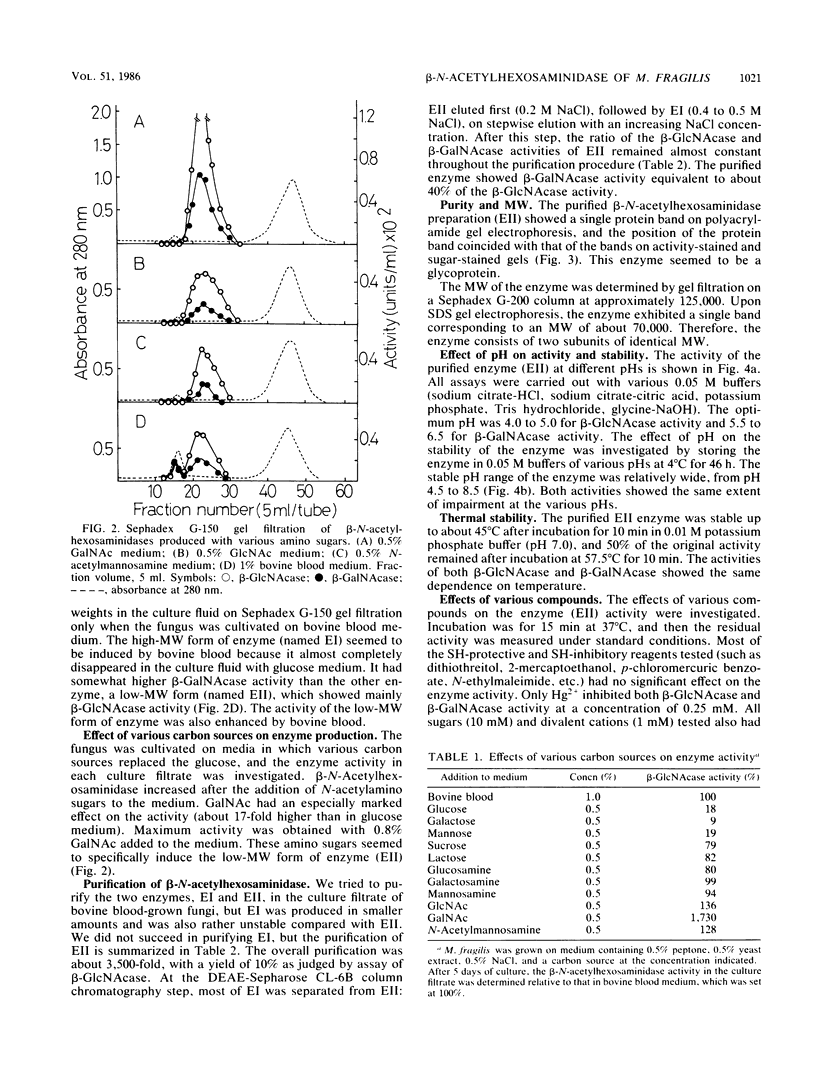

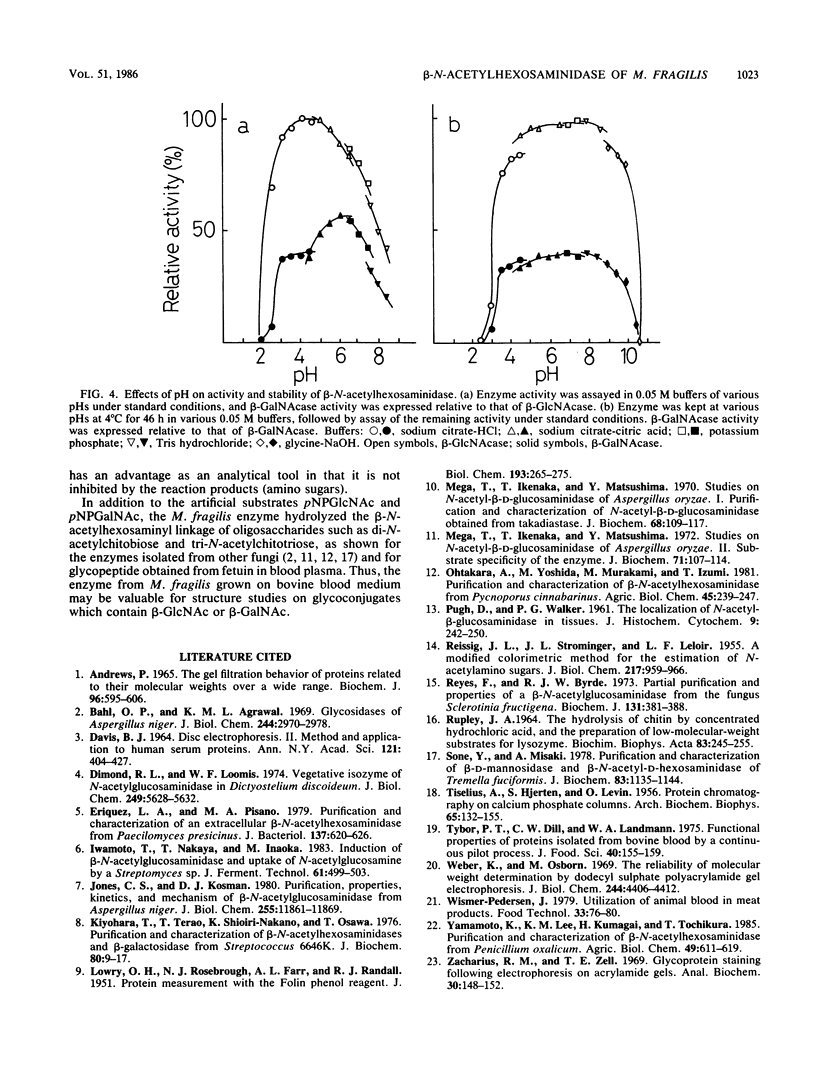
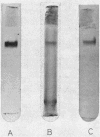
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl O. P., Agrawal K. M. Glycosidases of Aspergillus niger. I. Purification and characterization of alpha- and beta-galactosidases and beta-N-acetylglucosaminidase. J Biol Chem. 1969 Jun 10;244(11):2970–2978. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dimond R. L., Loomis W. F., Jr Vegetative isozyme of N-acetylglucosaminidase in Dictyostelium discoideum. J Biol Chem. 1974 Sep 10;249(17):5628–5632. [PubMed] [Google Scholar]
- Eriquez L. A., Pisano M. A. Purification and characterization of an extracellular beta-n-acetylhexosaminidase from Paecilomyces persicinus. J Bacteriol. 1979 Jan;137(1):620–626. doi: 10.1128/jb.137.1.620-626.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HJERTEN S., LEVIN O., TISELIUS A. Protein chromatography on calcium phosphate columns. Arch Biochem Biophys. 1956 Nov;65(1):132–155. doi: 10.1016/0003-9861(56)90183-7. [DOI] [PubMed] [Google Scholar]
- Jones C. S., Kosman D. J. Purification, properties, kinetics, and mechanism of beta-N-acetylglucosamidase from Aspergillus niger. J Biol Chem. 1980 Dec 25;255(24):11861–11869. [PubMed] [Google Scholar]
- Kiyohara T., Terao T., Shioiri-Nakano K., Osawa T. Purification and characterization of beta-N-acetylhexosaminidases and beta-galactosidase from Streptococcus 6646 K. J Biochem. 1976 Jul;80(1):9–17. doi: 10.1093/oxfordjournals.jbchem.a131263. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mega T., Ikenaka T., Matsushima Y. Studies on N-acetyl- -D-glucosaminidase of Aspergillus oryzae. II. Substrate specificity of the enzyme. J Biochem. 1972 Jan;71(1):107–114. doi: 10.1093/oxfordjournals.jbchem.a129731. [DOI] [PubMed] [Google Scholar]
- Mega T., Ikenaka T., Matsushima Y. Studies on N-acetyl-beta-D-glucosaminidase of Aspergillus oryzae. I. Purification and characterization of N-acetyl-beta-D-glucosaminidase obtained from Takadiastase. J Biochem. 1970 Jul;68(1):109–117. [PubMed] [Google Scholar]
- PUGH D., WALKER P. G. The localization of N-acetyl-beta-glucosaminidase in tissues. J Histochem Cytochem. 1961 May;9:242–250. doi: 10.1177/9.3.242. [DOI] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- RUPLEY J. A. THE HYDROLYSIS OF CHITIN BY CONCENTRATED HYDROCHLORIC ACID, AND THE PREPARATION OF LOW-MOLECULAR-WEIGHT SUBSTRATES FOR LYSOZYME. Biochim Biophys Acta. 1964 Nov 1;83:245–255. doi: 10.1016/0926-6526(64)90001-1. [DOI] [PubMed] [Google Scholar]
- Reyes F., Byrde R. J. Partial purification and properties of a beta-N-acetylglucosaminidase from the fungus Sclerotinia fructigena. Biochem J. 1973 Feb;131(2):381–388. doi: 10.1042/bj1310381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone Y., Misaki A. Purification and characterization of beta-D-mannosidase and beta-N-acetyl-D-hexosaminidase of Tremella fuciformis. J Biochem. 1978 Apr;83(4):1135–1144. doi: 10.1093/oxfordjournals.jbchem.a132003. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]