Abstract
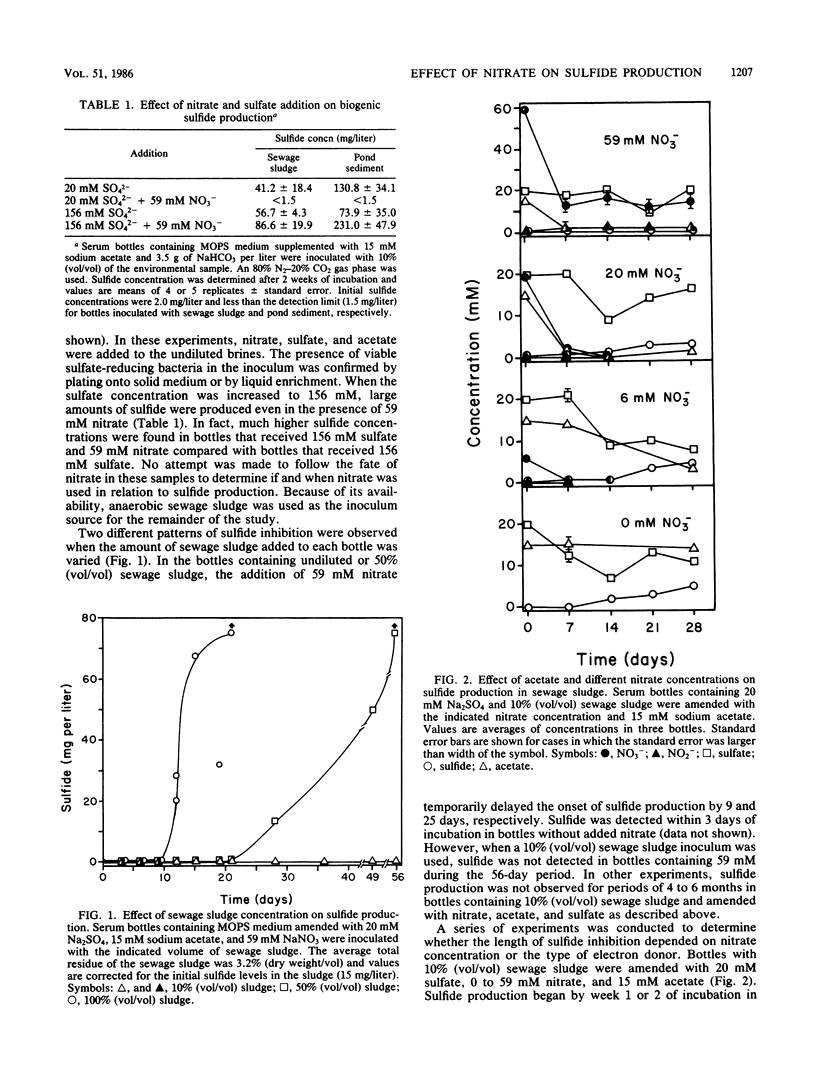
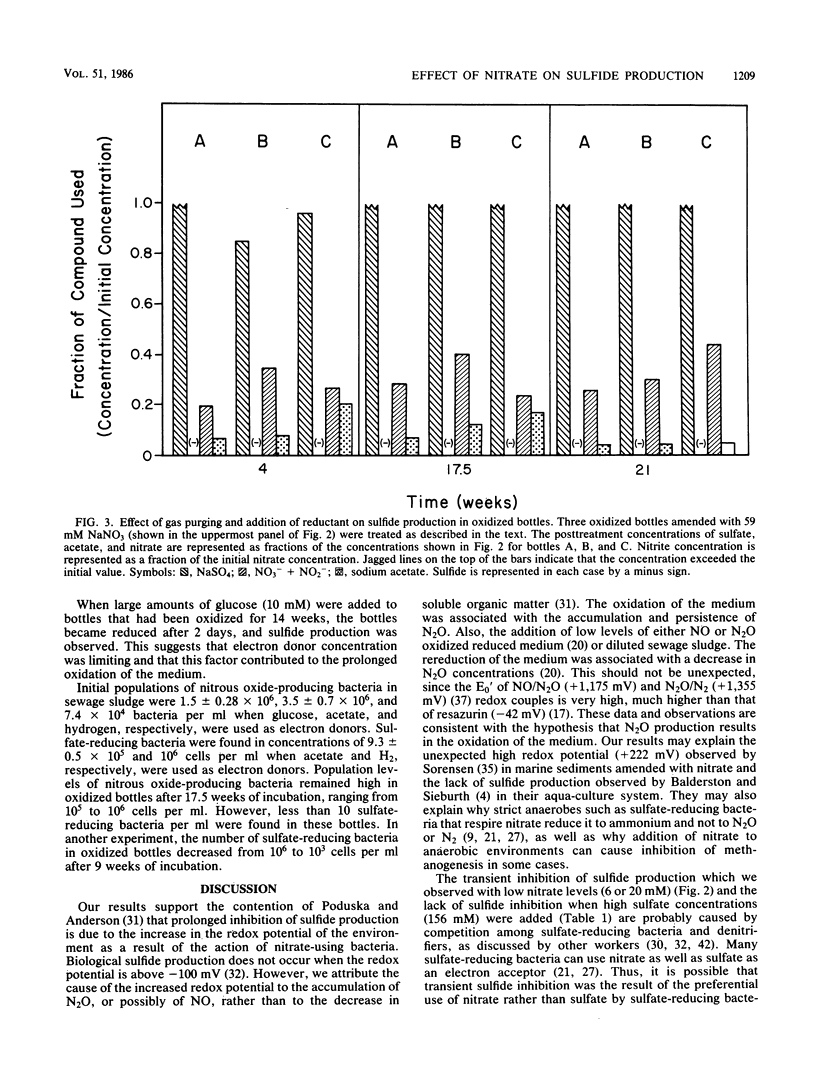
The addition of 59 mM nitrate inhibited biogenic sulfide production in dilute sewage sludge (10% [vol/vol]) amended with 20 mM sulfate and either acetate, glucose, or hydrogen as electron donors. Similar results were found when pond sediment or oil field brines served as the inoculum. Sulfide production was inhibited for periods of at least 6 months and was accompanied by the oxidation of resazurin from its colorless reduced state to its pink oxidized state. Lower amounts of nitrate (6 or 20 mM) and increased amounts of sewage sludge resulted in only transient inhibition of sulfide production. The addition of 156 mM sulfate to bottles with 59 mM nitrate and 10% (vol/vol) sewage sludge or pond sediment resulted in sulfide production. Nitrate, nitrite, and nitrous oxide were detected during periods where sulfide production was inhibited, whereas nitrate, nitrite, and nitrous oxide were below detectable levels at the time sulfide production began. The oxidation of resazurin was attributed to an increase in nitrous oxide which persisted in concentration of about 1.0 mM for up to 5 months. The numbers of sulfate-reducing organisms decreased from 106 CFU ml−1 sludge to less than detectable levels after prolonged incubation of oxidized bottles. The addition of 10 mM glucose to oxidized bottles after 14.5 weeks of incubation resulted in rereduction of the resazurin and subsequent sulfide production. The prolonged inhibition of sulfide production was attributed to an increase in oxidation-reduction potential due to biogenic production of nitrous oxide, which appeared to have a cytotoxic effect on sulfate-reducing populations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balderston W. L., Sieburth J. M. Nitrate removal in closed-system aquaculture by columnar denitrification. Appl Environ Microbiol. 1976 Dec;32(6):808–818. doi: 10.1128/aem.32.6.808-818.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betlach M. R., Tiedje J. M. Kinetic explanation for accumulation of nitrite, nitric oxide, and nitrous oxide during bacterial denitrification. Appl Environ Microbiol. 1981 Dec;42(6):1074–1084. doi: 10.1128/aem.42.6.1074-1084.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenneman G. E., Montgomery A. D., McInerney M. J. Method for detection of microorganisms that produce gaseous nitrogen oxides. Appl Environ Microbiol. 1986 Apr;51(4):776–780. doi: 10.1128/aem.51.4.776-780.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasna A. I., Rittenberg D. THE INHIBITION OF HYDROGENASE BY NITRIC OXIDE. Proc Natl Acad Sci U S A. 1954 Apr;40(4):225–227. doi: 10.1073/pnas.40.4.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera I., Dadák V., Dobrý R. The distribution of redox equivalents in the anaerobic respiratory chain of Paracoccus denitrificans. Eur J Biochem. 1983 Feb 1;130(2):359–364. doi: 10.1111/j.1432-1033.1983.tb07161.x. [DOI] [PubMed] [Google Scholar]
- Mancinelli R. L., McKay C. P. Effects of nitric oxide and nitrogen dioxide on bacterial growth. Appl Environ Microbiol. 1983 Jul;46(1):198–202. doi: 10.1128/aem.46.1.198-202.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J. Reduction of nitrogenous oxides by microorganisms. Bacteriol Rev. 1973 Dec;37(4):409–452. doi: 10.1128/br.37.4.409-452.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J. Denitrification rates in a marine sediment as measured by the acetylene inhibition technique. Appl Environ Microbiol. 1978 Jul;36(1):139–143. doi: 10.1128/aem.36.1.139-143.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J. Occurrence of nitric and nitrous oxides in a coastal marine sediment. Appl Environ Microbiol. 1978 Dec;36(6):809–813. doi: 10.1128/aem.36.6.809-813.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRUEPER H. G., SCHLEGEL H. G. SULPHUR METABOLISM IN THIORHODACEAE. I. QUANTITATIVE MEASUREMENTS ON GROWING CELLS OF CHROMATIUM OKENII. Antonie Van Leeuwenhoek. 1964;30:225–238. doi: 10.1007/BF02046728. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom S. R., Marquis R. E. Microbial growth modification by compressed gases and hydrostatic pressure. Appl Environ Microbiol. 1984 Apr;47(4):780–787. doi: 10.1128/aem.47.4.780-787.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedje J. M., Sexstone A. J., Myrold D. D., Robinson J. A. Denitrification: ecological niches, competition and survival. Antonie Van Leeuwenhoek. 1982;48(6):569–583. doi: 10.1007/BF00399542. [DOI] [PubMed] [Google Scholar]


