Abstract
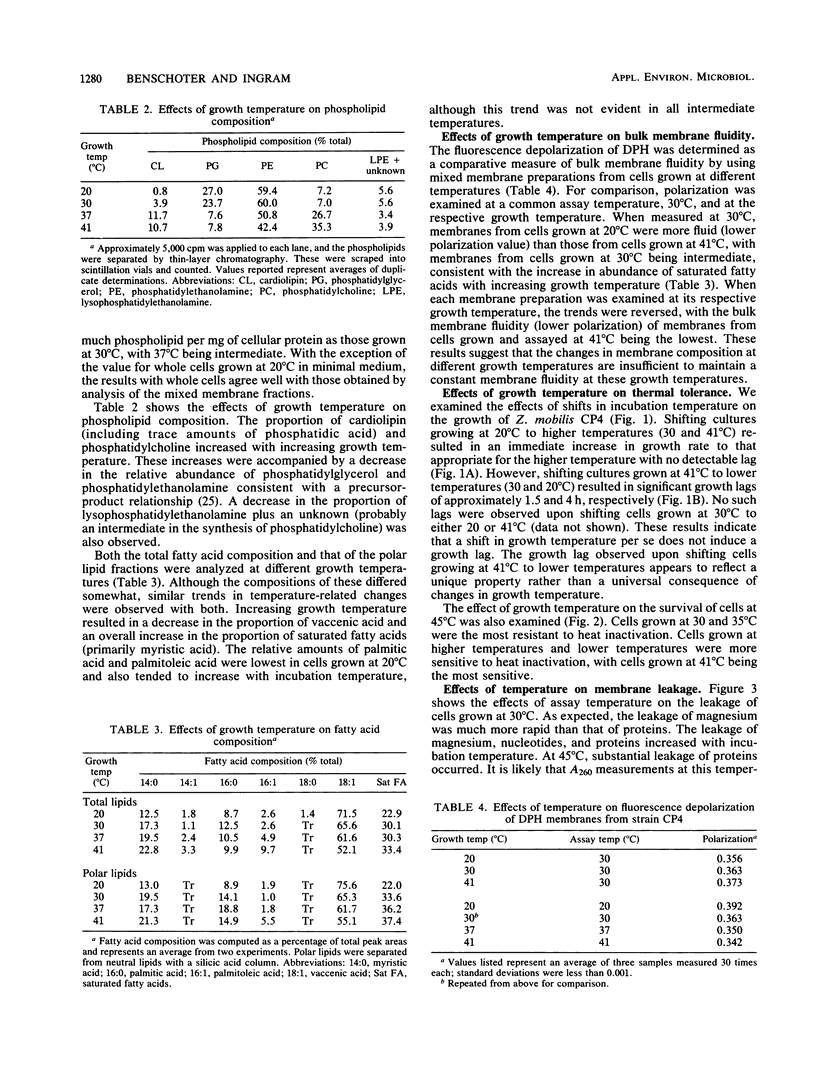
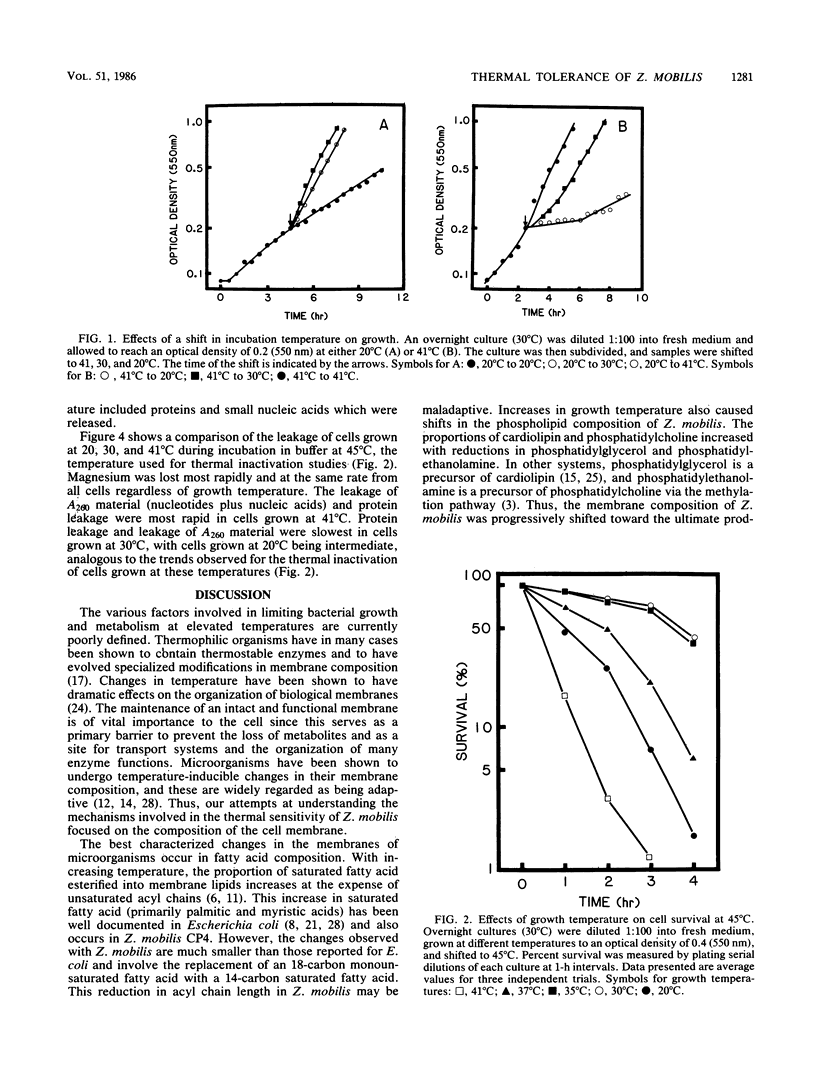
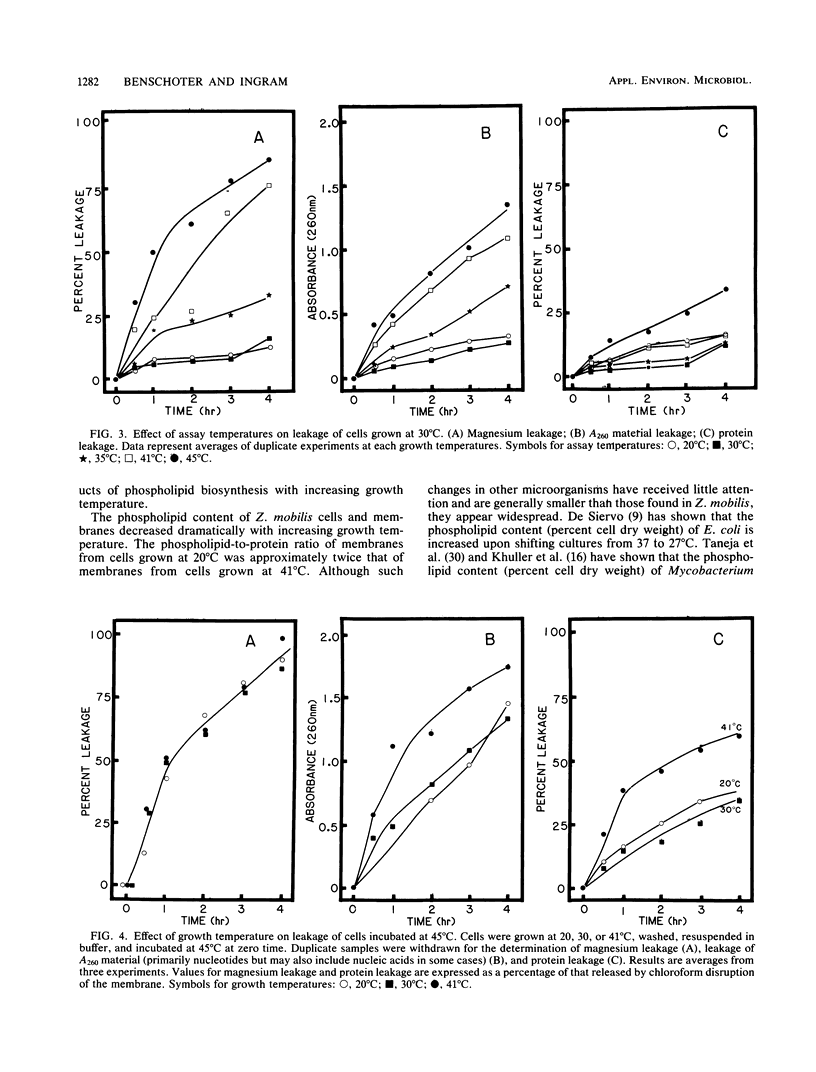
The membrane composition of Zymomonas mobilis changed dramatically in response to growth temperature. With increasing temperature, the proportion of vaccenic acid declined with an increase in myristic acid, the proportion of phosphatidylcholine and cardiolipin increased with decreases in phosphatidylethanolamine and phosphatidylglycerol, and the phospholipid/protein ratio of the membrane declined. These changes in membrane composition were correlated with changes in thermal tolerance and with changes in membrane fluidity. Cells grown at 20°C were more sensitive to inactivation at 45°C than were cells grown at 30°C, as expected. However, cells grown at 41°C (near the maximal growth temperature for Z. mobilis) were hypersensitive to thermal inactivation, suggesting that cells may be damaged during growth at this temperature. When cells were held at 45°C, soluble proteins from cells grown at 41°C were rapidly lost into the surrounding buffer in contrast to cells grown at lower temperatures. The synthesis of phospholipid-deficient membranes during growth at 41°C was proposed as being responsible for this increased thermal sensitivity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abruzzini A. F., Ingram L. O., Clem L. W. Temperature-mediated processes in teleost immunity: homeoviscous adaptation in teleost lymphocytes. Proc Soc Exp Biol Med. 1982 Jan;169(1):12–18. doi: 10.3181/00379727-169-41300. [DOI] [PubMed] [Google Scholar]
- Bell R. M., Coleman R. A. Enzymes of glycerolipid synthesis in eukaryotes. Annu Rev Biochem. 1980;49:459–487. doi: 10.1146/annurev.bi.49.070180.002331. [DOI] [PubMed] [Google Scholar]
- Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis: macromolecular synthesis in an sn-glycerol 3-phosphate acyltransferase Km mutant. J Bacteriol. 1974 Mar;117(3):1065–1076. doi: 10.1128/jb.117.3.1065-1076.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carey V. C., Ingram L. O. Lipid composition of Zymomonas mobilis: effects of ethanol and glucose. J Bacteriol. 1983 Jun;154(3):1291–1300. doi: 10.1128/jb.154.3.1291-1300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Vagelos P. R. Metabolism and function of the membrane phospholipids of Escherichia coli. Biochim Biophys Acta. 1972 Feb 14;265(1):25–60. doi: 10.1016/0304-4157(72)90018-4. [DOI] [PubMed] [Google Scholar]
- DAWSON R. M. A hydrolytic procedure for the identification and estimation of individual phospholipids in biological samples. Biochem J. 1960 Apr;75:45–53. doi: 10.1042/bj0750045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Siervo A. J. Alterations in the phospholipid composition of Escherichia coli B during growth at different temperatures. J Bacteriol. 1969 Dec;100(3):1342–1349. doi: 10.1128/jb.100.3.1342-1349.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis W. H., Yatvin M. B. Correlation of hyperthermic sensitivity and membrane microviscosity in E. coli K1060. Int J Radiat Biol Relat Stud Phys Chem Med. 1981 Mar;39(3):265–271. doi: 10.1080/09553008114550341. [DOI] [PubMed] [Google Scholar]
- Dombek K. M., Ingram L. O. Effects of ethanol on the Escherichia coli plasma membrane. J Bacteriol. 1984 Jan;157(1):233–239. doi: 10.1128/jb.157.1.233-239.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerty W. R., Makula R. A. Microbial lipid metabolism. CRC Crit Rev Microbiol. 1975 Oct;4(1):1–40. doi: 10.3109/10408417509105485. [DOI] [PubMed] [Google Scholar]
- Haest C. W., de Gier J., van Deenen L. L. Changes in the chemical and the barrier properties of the membrane lipids of E. coli by variation of the temperature of growth. Chem Phys Lipids. 1969 Dec;3(4):413–417. doi: 10.1016/0009-3084(69)90048-6. [DOI] [PubMed] [Google Scholar]
- Henning U., Dennert G., Rehn K., Deppe G. Effects of oleate starvation in a fatty acid auxotroph of Escherichia coli K-12. J Bacteriol. 1969 May;98(2):784–796. doi: 10.1128/jb.98.2.784-796.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter K., Rose A. H. Lipid composition of Saccharomyces cerevisiae as influenced by growth temperature. Biochim Biophys Acta. 1972 Apr 18;260(4):639–653. doi: 10.1016/0005-2760(72)90013-6. [DOI] [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. I. METABOLISM OF PHOSPHOLIPIDS IN ESCHERICHIA COLI B. J Biol Chem. 1963 Sep;238:2919–2922. [PubMed] [Google Scholar]
- Khuller G. K., Taneja R., Nath N. Effect of fatty acid supplementation on the lipid composition of Mycobacterium smegmatis ATCC 607, grown at 27 degrees and 37 degrees C. J Appl Bacteriol. 1983 Feb;54(1):63–68. doi: 10.1111/j.1365-2672.1983.tb01301.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marr A. G., Ingraham J. L. EFFECT OF TEMPERATURE ON THE COMPOSITION OF FATTY ACIDS IN ESCHERICHIA COLI. J Bacteriol. 1962 Dec;84(6):1260–1267. doi: 10.1128/jb.84.6.1260-1267.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre T. M., Chamberlain B. K., Webster R. E., Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis. Effects of cessation and reinitiation of phospholipid synthesis on macromolecular synthesis and phospholipid turnover. J Biol Chem. 1977 Jul 10;252(13):4487–4493. [PubMed] [Google Scholar]
- Quinn P. J., Chapman D. The dynamics of membrane structure. CRC Crit Rev Biochem. 1980;8(1):1–117. doi: 10.3109/10409238009105466. [DOI] [PubMed] [Google Scholar]
- Raetz C. R. Enzymology, genetics, and regulation of membrane phospholipid synthesis in Escherichia coli. Microbiol Rev. 1978 Sep;42(3):614–659. doi: 10.1128/mr.42.3.614-659.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert D. F., Ladenson R. C., Honegger J. L. The unsaturated fatty acid requirement in Escherichia coli. Temperature dependence and total replacement by branched-chain fatty acids. Biochim Biophys Acta. 1973 Jul 6;311(3):349–361. doi: 10.1016/0005-2736(73)90315-5. [DOI] [PubMed] [Google Scholar]
- Sinensky M. Homeoviscous adaptation--a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Feb;71(2):522–525. doi: 10.1073/pnas.71.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinensky M. Temperature control of phospholipid biosynthesis in Escherichia coli. J Bacteriol. 1971 May;106(2):449–455. doi: 10.1128/jb.106.2.449-455.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skotnicki M. L., Lee K. J., Tribe D. E., Rogers P. L. Comparison of ethanol production by different zymomonas strains. Appl Environ Microbiol. 1981 Apr;41(4):889–893. doi: 10.1128/aem.41.4.889-893.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja R., Malik U., Khuller G. K. Effect of growth temperature on the lipid composition of Mycobacterium smegmatis ATCC 607. J Gen Microbiol. 1979 Aug;113(2):413–416. doi: 10.1099/00221287-113-2-413. [DOI] [PubMed] [Google Scholar]


