Abstract
White clover is polymorphic for cyanogenesis, with both cyanogenic and acyanogenic plants occurring in nature. This chemical defense polymorphism is one of the longest-studied and best-documented examples of an adaptive polymorphism in plants. It is controlled by two independently segregating genes: Ac/ac controls the presence/absence of cyanogenic glucosides; and Li/li controls the presence/absence of their hydrolyzing enzyme, linamarase. Whereas Li is well characterized at the molecular level, Ac has remained unidentified. Here we report evidence that Ac corresponds to a gene encoding a cytochrome P450 of the CYP79D protein subfamily (CYP79D15), and we describe the apparent molecular basis of the Ac/ac polymorphism. CYP79D orthologs catalyze the first step in cyanogenic glucoside biosynthesis in other cyanogenic plant species. In white clover, Southern hybridizations indicate that CYP79D15 occurs as a single-copy gene in cyanogenic plants but is absent from the genomes of ac plants. Gene-expression analyses by RT–PCR corroborate this finding. This apparent molecular basis of the Ac/ac polymorphism parallels our previous findings for the Li/li polymorphism, which also arises through the presence/absence of a single-copy gene. The nature of these polymorphisms may reflect white clover's evolutionary origin as an allotetraploid derived from cyanogenic and acyanogenic diploid progenitors.
CYANOGENESIS (cyanide release following tissue damage) occurs in >2650 plant species, including ferns, gymnosperms, monocots, and dicots (Seigler and Brinker 1993). In its most common form, cyanogenesis involves the interaction of two compounds, cyanogenic glycosides and their hydrolyzing enzymes; these are separated in intact tissue and brought into contact with tissue disruption. White clover (Trifolium repens L., Fabaceae) is polymorphic for cyanogenesis, with both cyanogenic and acyanogenic plants occurring in natural populations (Armstrong et al. 1913; Ware 1925). This polymorphism arises through the presence/absence of both of the underlying cyanogenic components. Inheritance of cyanogenesis in white clover follows a simple Mendelian two-locus, two-allele model (Coop 1940; Melville and Doak 1940; Corkill 1942): the gene Ac/ac controls the presence/absence of cyanogenic glucosides; and the independently segregating gene Li/li controls the presence/absence of linamarase, a cyanogenic β-glucosidase (reviewed by Hughes 1991). Plants that carry at least one dominant (functional) allele at both genes (Ac and Li) are cyanogenic, while the occurrence of two nonfunctional alleles at either gene confers the acyanogenic phenotype.
White clover is a common legume of fields, lawns, and pastures in mesic temperate climates. It is a native of Eurasia but has been introduced worldwide as a forage crop and lawn plant. It is a perennial and an insect-pollinated, obligate outcrosser; plants also spread vegetatively by stolons. Chromosome number and genetic map data indicate that white clover is an allotetraploid (e.g., Barrett et al. 2004). While Ac and Li have not been mapped, both cyanogenesis genes are believed to be present in only one of the two parental genomes (e.g., Williams and Williamson 2001); this suggests that the species originated through the hybridization of a cyanogenic and an acyanogenic Trifolium species (Williams and Williamson 2001; Badr et al. 2002).
The ecological genetics of white clover cyanogenesis has been studied for >60 years, and this system represents one of the best-documented examples of an adaptive polymorphism in plants (reviews by Hughes 1991; Hayden and Parker 2002; Olsen et al. 2007). Cyanogenic plants are generally found to be strongly favored in the presence of generalist herbivores, which avoid eating them (e.g., Ennos 1981; Dirzo and Harper 1982a,b; Pederson and Brink 1998; Saucy et al. 1999; Viette et al. 2000). The factors favoring acyanogenic plants appear to be tied to resource allocation trade-offs and differential fitness in cool environments. Populations of white clover show strong clinal variation in cyanogenesis, with frequencies of cyanogenic plants closely correlated with minimum winter temperatures in both the native and the introduced range (e.g., Daday 1954a,b, 1958; De Araujo 1976; Till-Bottraud et al. 1988; Ganders 1990; Majumdar et al. 2004). Comparisons of cyanogenic and acyanogenic plants have revealed trade-offs between cyanogenesis and several fitness-related traits, including flowering rate (Daday 1965; Foulds and Grime 1972; Dirzo and Harper 1982b; Kakes 1989), drought tolerance (Foulds and Grime 1972), and resistance to rust (Dirzo and Harper 1982b). Thus, the predominance of acyanogenic plants in colder climates may reflect differential competitive ability under these conditions, possibly as a function of decreased herbivore abundance (Kakes 1989).
Understanding the genetic basis of adaptation is a major focus of modern evolutionary biology, and the white clover cyanogenesis polymorphism offers an attractive system for addressing this question. The simple Mendelian inheritance of the polymorphism makes it tractable for examining its molecular genetic basis and molecular evolution. At the same time, unlike polymorphisms in many model genetic systems (e.g., Arabidopsis thaliana, crop species), the ecology of this polymorphism is very well studied; >50 articles on white clover cyanogenesis have been published since the 1940s, and the system has become a textbook example of variation maintained by opposing selective forces (e.g., Dirzo and Sarukhan 1984; Silvertown and Charlesworth 2001). This ecological foundation makes it possible to more fully understand the ecological and evolutionary relevance of underlying molecular genetic variation.
In a previous study (Olsen et al. 2007), we examined the molecular genetic basis and population genetics of the Li/li polymorphism, which is responsible for the presence/absence of linamarase. In the present study, we extend our molecular evolutionary analysis to the Ac gene, which heretofore has remained unidentified at the molecular level. We address (1) the molecular identity of Ac; (2) the molecular genetic basis of the Ac/ac polymorphism, underlying the presence/absence of cyanogenic glucosides in white clover plants; and (3) the molecular population genetics of Ac, as inferred through comparisons of DNA sequences at this gene and three unlinked, neutrally evolving genes.
Our analysis of Ac/ac is based on a well-established foundation of information on plant cyanogenic glucoside biosynthesis. This pathway has been studied extensively in several species, including the model legume Lotus japonicus (Forslund et al. 2004; reviewed by Bak et al. 2006), which is in the same subfamily as white clover. Plant cyanogenic glucosides are synthesized from amino acids in a highly channeled set of reactions catalyzed by two membrane-bound cytochrome P450's and a soluble glycosyltransferase (Forslund et al. 2004; Bak et al. 2006). These three proteins are believed to form a metabolon (multienzyme complex) that acts to channel the otherwise toxic and highly reactive intermediates through the pathway to the formation of the end product (Bak et al. 2000, 2006; Kristensen et al. 2005). Cyanogenic white clover plants (as well as L. japonicus, cassava, lima bean, flax, and rubber tree) synthesize two closely related cyanogenic glucosides: linamarin (1-cyano-1-methylethyl β-d-glucopyranoside) and lotaustralin (R-1-cyano-1-methylpropyl β-d-glucopyranoside); these are generated from valine and isoleucine, respectively (Butler and Butler 1960). A single metabolon is responsible for the synthesis of both forms of cyanogenic glucoside (Collinge and Hughes 1984; Forslund et al. 2004). White clover plants that possess only nonfunctional ac alleles are unable to synthesize either linamarin or lotaustralin (Corkill 1942; Hughes and Conn 1976).
The highly reactive nature of intermediates in the cyanogenic glucoside biosynthesis pathway suggests that the ac phenotype would be very unlikely to arise through the disruption of an intermediate step in the pathway, as this would lead to the accumulation of toxic intermediates (Bak et al. 1999, 2000; Kristensen et al. 2005). In L. japonicus and cassava, the first step in the pathway is catalyzed by a cytochrome P450 belonging to the CYP79D protein subfamily (Andersen et al. 2000; Forslund et al. 2004). Therefore, our hypothesis in conducting the present study was that a gene encoding a white clover CYP79D protein (or, alternatively, a gene controlling the expression of this protein) is responsible for the Ac/ac polymorphism.
MATERIALS AND METHODS
Samples:
White clover's current cosmopolitan distribution represents a recent and rapid population expansion, both in the Old World (following the development of agriculture within the last 10,000 years) and worldwide (following European colonization within the last 500 years). Seeds from a geographically diverse sample of wild T. repens populations were obtained from the white clover germ plasm collection maintained by the U.S. Department of Agriculture (http://www.ars-grin.gov). Forty-two white clover samples were included in the study, representing 26 Ac plants (synthesizing cyanogenic glucosides) and 16 ac plants (lacking cyanogenic glucosides) (Table 1). Seeds were lightly scarified, germinated, and grown in standard greenhouse conditions at Washington University. A single plant was grown per U.S. Department of Agriculture (USDA) accession number. Two related, cyanogenic Trifolium species were also included for use as outgroups in DNA sequence analyses (Table 1).
TABLE 1.
Trifolium samples used in analyses
| Trifolium species | USDA accession | Country of origin | Ac/ac phenotype | Li/li phenotype | CYP79D15 haplotype |
|---|---|---|---|---|---|
| T. repens | PI 100247ab | New Zealand | Ac | li | 2/14 |
| T. repens | PI 200372abc | Israel | Ac | Li | 2/4 |
| T. repens | PI 204930abc | Turkey | Ac | Li | 9/10 |
| T. repens | PI 205062ab | Turkey | Ac | li | 11/12 |
| T. repens | PI 214207ab | Israel | Ac | Li | 1/12 |
| T. repens | PI 217444ab | Italy | Ac | Li | 1/12 |
| T. repens | PI 221961ab | Afghanistan | Ac | li | 13/13 |
| T. repens | PI 226996ab | Uruguay | Ac | Li | 1/3 |
| T. repens | PI 230183b | Argentina | Ac | Li | 14/15 |
| T. repens | PI 234678b | France | Ac | Li | 1/5 |
| T. repens | PI 239977abc | Portugal | Ac | Li | 1/4 |
| T. repens | PI 246751ab | Spain | Ac | Li | 1/1 |
| T. repens | PI 260646ab | Greece | Ac | Li | 1/4 |
| T. repens | PI 291828a | Chile | Ac | Li | — |
| T. repens | PI 294546ab | France | Ac | Li | 2/2 |
| T. repens | PI 298485abc | Israel | Ac | Li | 1/1 |
| T. repens | PI 302441ab | Australia | Ac | li | 1/12 |
| T. repens | PI 311490a | Spain | Ac | Li | — |
| T. repens | PI 311494ab | Spain | Ac | Li | 1/12 |
| T. repens | PI 315542b | Russia | Ac | li | 5/5 |
| T. repens | PI 345529ab | Australia | Ac | Li | 7/8 |
| T. repens | PI 440745ab | Russia | Ac | li | 13/13 |
| T. repens | PI 440746a | Russia | Ac | li | — |
| T. repens | PI 499685a | China | Ac | li | — |
| T. repens | PI 499688ab | China | Ac | li | 13/13 |
| T. repens | PI 597530b | Lithuania | Ac | li | 1/6 |
| T. repens | PI 195534a | Italy | ac | li | — |
| T. repens | PI 208730a | Italy | ac | li | — |
| T. repens | PI 232109ac | Germany | ac | li | — |
| T. repens | PI 251053a | Macedonia | ac | li | — |
| T. repens | PI 251190a | Serbia | ac | li | — |
| T. repens | PI 251191a | Yugoslavia | ac | li | — |
| T. repens | PI 251197ac | Bosnia | ac | li | — |
| T. repens | PI 253323a | Slovenia | ac | Li | — |
| T. repens | PI 282378ac | Italy | ac | li | — |
| T. repens | PI 419314a | Greece | ac | li | — |
| T. repens | PI 494747a | Romania | ac | li | — |
| T. repens | PI 516411a | Romania | ac | li | — |
| T. repens | PI 542904a | Croatia | ac | li | — |
| T. repens | PI 542905a | Croatia | ac | Li | — |
| T. repens | PI 542915a | Bosnia | ac | li | — |
| T. repens | PI 556991ac | United States | ac | li | — |
| T. nigrescens ssp. petrisavii | PI 120103b | Turkey | Ac | Li | — |
| T. nigrescens ssp. petrisavii | PI 298478b | Israel | Ac | Li | — |
| T. isthmocarpum | PI 422595b | Morocco | Ac | Li | — |
CYP79D15 haplotype labels correspond to those in Table 2.
Analyzed by Southerns.
Analyzed by CYP79D15 DNA sequencing.
Analyzed by RT–PCR.
Plants were assayed for the presence/absence of cyanogenic constituents using a modified Feigl–Anger hydrogen cyanide (HCN) assay (Feigl and Anger 1966), as described by Olsen et al. (2007). The presence/absence of functional alleles at Ac and Li can be inferred independently through the exogenous addition of either linamarase or linamarin, respectively, to an acyanogenic leaf sample (see Olsen et al. 2007). Liberated cyanide is normally detectable in the Feigl–Anger assay within 1 hr of leaf tissue incubation with test reagents. Because we observed wide quantitative variation in cyanogenic glucoside content among Ac plant samples (discussed below), all leaf samples were incubated with HCN test reagents for ≥3 hr to ensure detection of all Ac genotypes.
PCR amplification and DNA sequencing:
DNA was extracted from fresh leaf tissue using a modified CTAB extraction protocol (Porebski et al. 1997). In L. japonicus, two independent cyanogenic glucoside biosynthetic pathways operate in roots and in aerial parts of the plant; each pathway uses a different CYP79D enzyme (Forslund et al. 2004). The genes encoding these two enzymes have been cloned (CYP79D3, CYP79D4; Forslund et al. 2004), and comparison of their cDNA sequences reveals that they are 96% identical. We designed degenerate primers from these L. japonicus CYP79D genes to PCR amplify any T. repens homologs. Initial PCR amplification was performed using the following primers: 5′-TGGACTTTTTTGCTTGTTGTGATATT-3′ and 5′-GCAGCCAATCTTGGTTTTGC-3′, located at the 5′ and 3′ ends, respectively, of the L. japonicus full-length cDNAs (see supplemental Tables S1 and S2). We subsequently designed several additional primers from T. repens sequences for use in PCR and DNA sequencing (supplemental Table S1).
PCR was performed with 20-μl volumes and standard reaction conditions, using GoTaq DNA polymerase (Promega, Madison, WI). Annealing temperatures were adjusted for primer combinations. PCR products were cloned into plasmids using TA cloning (Invitrogen, Carlsbad, CA), purified, and then sequenced. A minimum of eight clones were sequenced per PCR product to detect allelic variation and to allow detection of PCR artifacts. Singletons unique to a single sequenced clone were considered artifacts of polymerase error and disregarded. PCR using CYP79D primers consistently amplified a single product in Ac plants, which DNA sequencing confirmed to be a T. repens CYP79D ortholog. This T. repens gene is hereafter referred to as CYP79D15, in accordance with cytochrome P450 nomenclatural convention (http://drnelson.utmem.edu/cytochromeP450.html).
CYP79D15 was sequenced in 22 Ac plants. For outgroup comparison in DNA sequence analyses, orthologs of CYP79D15 were also amplified and sequenced in two closely related cyanogenic clover species: T. nigrescens ssp. petrisavii and T. isthmocarpum (Table 1). All DNA sequencing was performed using BigDye terminators (Applied Biosystems, Foster City, CA), with reactions run on an ABI 3130 sequencer in the Biology Department of Washington University. No more than two sequence haplotypes (alleles) were detected per individual, an indication that paralogous or homeologous gene copies were not amplified or included in sequence analyses.
Determination of gene copy number:
We performed Southern hybridizations to assess CYP79D15 copy number in Ac and ac plants and to detect any other genes sharing sequence similarity with CYP79D15. Twenty-two Ac plants and 16 ac plants were sampled for Southern hybridizations (Table 1). Genomic DNA was purified as described above, and ∼1 μg DNA was digested with AflIII or AseI and run on a 0.6% Seakem (Fisher Scientific, Pittsburgh) agarose gel. The DNA was then transferred to a nitrocellulose membrane using standard methods. A probe corresponding to the middle portion of CYP79D15 (892 bp, spanning 52% of the gene) was prepared using the DIG probe synthesis kit (Roche, Indianapolis); hybridization washes were performed at high stringency following the manufacturer's protocol. Primers used in PCR amplification of probes are listed in supplemental Table S1. On the basis of the DNA sequence of CYP79D15, AseI is predicted to lack restriction sites within the gene; AflIII is predicted to have two restriction sites within the gene, one of which occurs within the probed region.
Gene-expression analysis:
To assess CYP79D15 expression in Ac and ac plants, total RNA was extracted from fresh young leaf tissue of four Ac and four ac plant samples (Table 1), using RNeasy kits (QIAGEN, Valencia, CA). Reverse transcription and RT–PCR were performed using a Thermoscript RT–PCR kit (Invitrogen). The CYP79D15 primers that were used to amplify a probe for Southern hybridizations were also used in RT–PCR. These primers are predicted to amplify a 752-bp portion of cDNA corresponding to the 892-bp genomic DNA sequence. As a positive control for RT–PCR, primers were designed to amplify a 151-bp region of 5.8S ribosomal RNA. All RT–PCR primers are listed in supplemental Table S1. PCR conditions were similar to those for amplifying genomic DNA as described above, with annealing temperatures adjusted for primer combinations. The identities of all RT–PCR products were confirmed by DNA sequencing.
DNA sequence analyses:
DNA sequences from CYP79D15 were edited and aligned visually using Biolign software (Hall 2001). The position of a single intron within the gene was inferred by comparison to published L. japonicus CYP79D cDNA sequences (see supplemental Table S2). Molecular population genetic analyses were conducted using DnaSP 4.10 (Rozas et al. 2003). Levels of nucleotide diversity per silent site were estimated as π (Nei 1987) and θW (Watterson 1975). Tests of selection were performed using Tajima's (1989) D and Fay and Wu's (2000) H-tests, with statistical significance assessed by coalescent simulations (1000 replicates, using θW and levels of recombination estimated empirically from the data) and by the McDonald–Kreitman (McDonald and Kreitman 1991) test.
Diversity measures and tests of selection for CYP79D15 were compared to previously published data sets for three other T. repens loci (ACO1, ALDP, ZIP; Olsen et al. 2007). These three genes have been sequenced in 18–20 white clover samples from across the species range; approximately half of the plant samples sequenced at each of the genes were also included in the present study (see Olsen et al. 2007). Sequences from all three previously published genes conform to neutral equilibrium expectations in tests of selection and are considered here to be representative of neutrally evolving white clover genes. Analyses of population structure using these three neutral loci have revealed no evidence of geographical structuring of genetic variation across the present species range (Olsen et al. 2007), a pattern consistent with the recent and rapid dispersal of this cosmopolitan, human-associated plant.
RESULTS
CYP79D15 gene structure and copy number:
Degenerate PCR primers designed from the ends of L. japonicus CYP79D full-length cDNAs amplified a single 1.7-kb product in white clover plants that possess cyanogenic glucosides (Ac plants). The structure of the amplified gene is shown in Figure 1. Exon regions are 92–93% identical to two previously identified L. japonicus CYP79 genes (CYP79D3, CYP79D4; Forslund et al. 2004), and the inferred amino acid sequence is 89–90% identical. Following the conventions of the cytochrome P450 nomenclature committee (http://drnelson.utmem.edu/cytochromeP450.html), the white clover gene has been designated CYP79D15. Whereas L. japonicus expresses two tissue-specific isoforms of CYP79D, with one (CYP79D3) expressed exclusively in aerial parts and the other (CYP79D4) expressed in roots (Forslund et al. 2004), only a single gene encoding a CYP79D protein was detected in white clover by PCR; this result is also supported by Southern hybridizations (discussed below). White clover synthesizes cyanogenic glucosides exclusively in shoot growth (Collinge and Hughes 1982b); therefore, the apparent absence of multiple tissue-specific CYP79D isoforms is not unexpected.
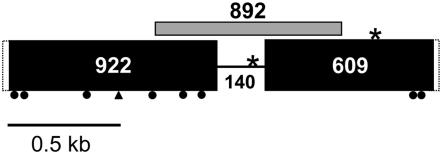
Figure 1.—
Structure of the CYP79D15 gene. Dotted rectangles at 5′ and 3′ ends of the gene indicate the boundaries of the full-length cDNA (start codon to stop codon) as inferred by alignment with Lotus japonicus CYP79D cDNAs (see supplemental Table S2); unshaded regions at the 5′ and 3′ ends were not included in sequence analyses. Solid rectangles represent sequenced exon regions, and the intervening line corresponds to the single intron. Sizes of exons and the intron are approximately to scale; numbers indicate aligned nucleotide lengths of each sequenced gene region. Solid dots indicate approximate positions of nonsynonymous substitutions; the triangle indicates the approximate location of a four- to five-codon indel (see Table 2). Asterisks indicate predicted AflIII restriction sites. The shaded bar indicates the gene region targeted for Southern hybridizations and RT–PCR.
Whereas CYP79D15 was consistently amplified in Ac plants (possessing cyanogenic glucosides), we were unable to amplify any portion of the gene in ac plants, despite repeated attempts using multiple primer combinations (see supplemental Table S1) in 16 different ac genotypes. We therefore performed Southern hybridizations to assess CYP79D15 copy number and to detect any related genes, using a sample of 22 Ac and 16 ac plants (Table 1). Figure 2 shows hybridizations for a representative sample of Ac and ac plants digested with AseI, which has no predicted restriction sites within CYP79D15. A single strong band is observed in AseI digests of Ac plants, consistent with the occurrence of CYP79D15 as a single-copy gene in Ac plants. In contrast, no bands are detectable for the ac samples, indicating the absence of sequences with close sequence similarity to the CYP79D15 probe. Similarly, AflIII digests, which are predicted to generate two bands for a single-copy gene, reveal two bands in Ac plants and no bands in ac plants (supplemental Figure S1). These same banding patterns were observed consistently in all Ac and ac plants examined (data not shown). Thus, for this worldwide sample of white clover plants, the Ac phenotype is consistently associated with the occurrence of CYP79D15 as a single-copy gene, and the ac phenotype is associated with the absence of much or all of the gene from the white clover genome. In addition, the lack of hybridization to more than one gene copy (Figure 2) indicates that there is no evidence for any additional T. repens genes with close sequence similarity to CYP79D15.
Figure 2.—
Southern hybridizations of Ac and ac white clover samples digested with AseI and hybridized with a CYP79D15-specific probe. Ac plants (possessing cyanogenic glucosides) and ac plants (lacking cyanogenic glucosides) are labeled + and −, respectively. The CYP79D15 probe corresponds to an 892-bp portion of genomic DNA sequence spanning approximately half of the gene (see Figure 1). AseI has no predicted restriction sites within CYP79D15. The two hybridizations shown (A and B) include partial replication of samples, as follows: (A) Ac accessions (left to right) 260646, 291828, 302441, 311494 and ac accessions 251053, 251190, 542915, 556991; (B) Ac accessions 246751, 260646, 291828, 302441 and ac accessions 195534, 232019, 516411, 556991.
CYP79D15 expression in Ac and ac plants:
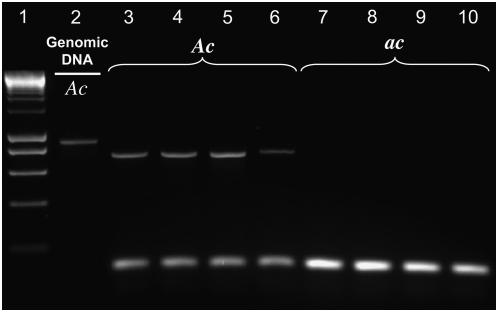
RT–PCR primers were designed to amplify a 752-bp portion of CYP79D15 cDNA, corresponding to a genomic DNA region of 892 bp in the center of the gene (see Figure 1). CYP79D15 transcripts were detected in all four of the Ac plants examined and not in any of the four ac plants (Figure 3); DNA sequencing of the Ac RT–PCR products confirmed that they correspond to targeted exon portions of CYP79D15. The absence of CYP79D15 expression in ac plants is consistent with the apparent absence of much or all of the gene from the genomes of ac plants (see above; Figure 2).
Figure 3.—
Expression of CYP79D15 in Ac and ac white clover plants. The targeted gene region is indicated in Figure 1. Lane 1 (ladder), first five bands from bottom: 200 bp, 400 bp, 600 bp, 800 bp, 1000 bp. Lane 2 (genomic DNA control): CYP79D15 PCR from genomic DNA of Ac accession PI 239977; the predicted product size is 892 bp. Lanes 3–10: CYP79D15 and 5.8S rDNA PCR products from cDNA of Ac accessions (lanes 3–6, accessions 239977, 202372, 298485, 204930) and ac accessions (lanes 7–10, accessions 556991, 232109, 251197, 282378); the predicted product sizes are 752 bp for CYP79D15 (corresponding to 892 bp minus the 140-bp intron) and 151 bp for the 5.8S rDNA.
DNA sequence variation:
CYP79D15 was sequenced in 22 Ac plants representing a geographically widespread sample of the species range (Table 1). Previous analyses of neutral genetic variation have indicated no evidence of population substructure in white clover (Olsen et al. 2007; see above). Fourteen substitution polymorphisms were observed at CYP79D15 (5 synonymous; 8 nonsynonymous, encoding seven amino acid replacements; 1 noncoding); in addition, indels were observed both in the intron (seven regions of indels, ranging in size from 1 to 28 bp) and in exon 1, where an in-frame indel codes for insertions of four- or five-amino-acid residues downstream of Asp164 (Table 2). Observed polymorphisms characterize a total of 15 CYP79D15 sequence haplotypes (Tables 1 and 2). No frameshift mutations, premature stop codons, or other polymorphisms were observed that would be predicted to lead to a loss of gene function. Tests of selection do not reveal any statistically significant deviations from neutral equilibrium (Tajima's D = −0.8514, P > 0.1; Fay and Wu's H = 1.59, P > 0.1; McDonald–Kreitman test, P > 0.1 by Fisher's exact test). This result is observed regardless of whether all Ac plants are treated as AcAc homozygotes (i.e., possessing two CYP79D15 sequences; test results reported here) or whether the accessions that possess only one CYP79D15 haplotype (six accessions; see Table 1) are treated as hemizygotes (i.e., contributing only one CYP79D15 sequence; test results not shown).
TABLE 2.
Nucleotide substitution polymorphisms and coding-region indels at CYP79D15
| Exon 1
|
Intron: | Exon 2
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Haplotype | No. | 29a | 59a | 280 | 319 | 384a | 497b | 746a | 860a | 903a | 933 | 1385 | 1400 | 1517 | 1638a | 1639a |
| 1 | (13) | T | C | T | T | G | — | G | A | T | A | A | C | T | C | C |
| 2 | (4) | . | . | . | . | . | — | . | . | A | . | . | . | . | . | . |
| 3 | (1) | . | . | . | A | . | — | . | . | . | . | . | . | . | . | . |
| 4 | (3) | . | . | . | A | . | — | . | . | A | . | . | . | . | . | . |
| 5 | (3) | . | . | . | A | . | — | . | . | A | . | . | T | . | A | T |
| 6 | (1) | . | . | . | A | . | — | . | . | A | . | . | T | . | . | . |
| 7 | (1) | . | . | . | A | A | 5 | A | . | A | . | G | . | . | . | . |
| 8 | (1) | A | T | . | A | A | 5 | . | . | A | . | G | . | . | . | . |
| 9 | (1) | . | . | . | A | A | 4 | . | . | A | . | . | . | . | A | T |
| 10 | (1) | . | . | . | A | A | 4 | . | . | A | T | . | . | . | A | T |
| 11 | (1) | . | . | . | A | A | 4 | . | . | A | T | . | . | C | A | T |
| 12 | (5) | . | . | . | A | . | 4 | . | . | A | . | . | . | . | . | . |
| 13 | (6) | . | . | . | A | . | 4 | . | . | A | T | . | . | . | A | T |
| 14 | (2) | . | . | . | A | . | 4 | . | . | . | . | . | . | . | . | . |
| 15 | (1) | . | . | C | A | . | 4 | . | G | . | . | . | . | . | . | . |
Numbers in parentheses indicate the number of sequences observed per haplotype. Dots indicate that a nucleotide is identical to that of haplotype 1. Nucleotide positions 1638 and 1639 correspond to a single codon.
Nonsynonymous polymorphisms.
4, four-codon insertion after Asp164 (Val-Asn-Asp-Asp); 5, five-codon insertion after Asp164 (Val-Asn-Asp-Asp-Asp).
CYP79D15 nucleotide diversity estimates are comparable between nonsynonymous sites (π = 0.0013, θW = 0.0016) and silent sites (π = 0.0013, θW = 0.0029). Comparison of these values with those of three previously examined neutral genes (ACO1, ALDP, ZIP; Olsen et al. 2007) suggests at face value that CYP79D15 has much lower levels of genetic diversity; mean silent-site nucleotide diversity for the three previously published loci is π = 0.0142 ± 0.0061 and θW = 0.0113 ± 0.0056. The lower nucleotide diversity at CYP79D15 is partly a reflection of overlapping indel and substitution polymorphisms within the intron, which creates an underestimate of nucleotide variation; recoding indels as single-character substitutions increases the estimates of nucleotide diversity (π = 0.0053, θW = 0.0081). In addition, because CYP79D15 is absent in ac alleles (see above), the smaller effective population size of this gene must also be taken into account in comparisons with other autosomal nuclear loci. If one assumes that Ac and ac alleles occur at roughly equal frequencies within the species (see, e.g., Daday 1958), such that the effective population size of CYP79D15 is roughly half that of the other nuclear genes, then the recalibrated estimate of silent-site nucleotide diversity (π = 0.01) is comparable to that of the three previously examined neutral genes. Together with the nonsignificant tests of selection, this pattern suggests that CYP79D15 is evolving in a manner consistent with neutral equilibrium expectations.
DISCUSSION
CYP79D15 and the Ac/ac polymorphism:
The white clover cyanogenesis polymorphism was first identified >90 years ago (Armstrong et al. 1913; Ware 1925), and for the last half century this system has served as a model for understanding the ecological genetics of adaptive polymorphism in natural populations (reviewed by Hughes 1991; Hayden and Parker 2002). Although Li and its product have been well characterized at the nucleotide and protein levels (Oxtoby et al. 1991; Barrett et al. 1995; Olsen et al. 2007), the molecular identity of Ac has remained undetermined. Below we discuss evidence that Ac is very likely to be the cytochrome P450 gene CYP79D15.
-
Ac/ac is predicted to occur at the first step in cyanogenic glucoside biosynthesis. The biosynthesis of cyanogenic glucosides occurs in a highly channeled set of reactions catalyzed by three proteins, and interruption of the pathway at points other than the first dedicated step leads to the accumulation of reactive, toxic intermediates (reviewed by Bak et al. 2006). This has been demonstrated elegantly in metabolic engineering experiments, in which acyanogenic species (A. thaliana, Nicotiana tabacum) have been transformed to express components of the cyanogenic glucoside biosynthesis pathway from Sorghum bicolor (Bak et al. 1999, 2000; Kristensen et al. 2005). When A. thaliana is transformed to express the entire S. bicolor cyanogenic glucoside pathway, it is morphologically normal, while accumulating 4% dry-weight cyanogenic glucosides; in contrast, plants expressing only the first two steps in the pathway show severe stunting and metabolic evidence of detoxification reactions (Kristensen et al. 2005). Similarly, transgenic N. tabacum plants expressing only the first step of the S. bicolor pathway show evidence of intoxication from reactive intermediates (Bak et al. 2000). These transgenic studies strongly suggest that for white clover, the first dedicated step in the pathway would be most likely to account for the Ac/ac polymorphism.
Additional support for this hypothesis comes from in vivo and in vitro labeling experiments conducted in white clover prior to the development of the current metabolon model (Hughes and Conn 1976; Collinge and Hughes 1982a). These studies indicate that ac plants entirely lack the first step in the pathway while showing weak but measurable activity in subsequent steps (e.g., Collinge and Hughes 1982a).
CYP79D15 catalyzes the first step in white clover cyanogenic glucoside biosynthesis. Our data strongly suggest that this first step in white clover cyanogenic glucoside biosynthesis is catalyzed by the gene product of CYP79D15. CYP79D proteins catalyze the first step in this pathway in both of the linamarin/lotaustralin-producing species that have been examined previously (cassava and L. japonicus) (Andersen et al. 2000; Forslund et al. 2004). In white clover, PCR using degenerate primers from L. japonicus amplifies a single gene belonging to the CYP79D protein subfamily (Figures 1 and 3). This gene, CYP79D15, shows very high sequence similarity to L. japonicus CYP79D orthologs, not only at the inferred amino acid level (≥89% identical), but also at the nucleotide level (≥92% identical) (see supplemental Table S2); thus, there is little question as to its placement within the CYP79D protein subfamily (see criteria described by Nelson 2006). Moreover, PCR amplifications and Southern hybridizations both indicate that there are no other genes present in the white clover genome with close sequence similarity to CYP79D15 (Figures 1 and 2). Taken together, these observations suggest that CYP79D15 is the only obvious candidate to catalyze the first step in white clover cyanogenic glucoside biosynthesis and as such would be the most likely candidate to underlie the Ac/ac cyanogenic glucoside polymorphism.
CYP79D15 molecular variation corresponds to the Ac/ac phenotype. Additional evidence that CYP79D15 corresponds to Ac comes from our analysis of CYP79D15 molecular variation. In Southern hybridizations using a worldwide sample of white clover plants (26 Ac plants, 16 ac plants), we find a perfect correlation between the presence of CYP79D15 in the white clover genome and the synthesis of cyanogenic glucosides (Figure 2; Table 1). Consistent with this finding, we also find a perfect correlation between the presence of cyanogenic glucosides and CYP79D15 gene expression in leaf tissue (Figure 3). Given that CYP79D15 is the obvious candidate to catalyze the first step in cyanogenic glucoside biosynthesis, these findings strongly suggest that the Ac/ac polymorphism arises through the presence/absence of CYP79D15 in the white clover genome.
As a further examination of CYP79D15 and the Ac/ac polymorphism, we assessed phenotypic and genetic variation in an independent sample of 48 white clover plants collected in the vicinity of Washington University (St. Louis). Plants were scored for the presence/absence of cyanogenic glucosides, the presence/absence of CYP79D15 (as assayed by PCR), and SNP variation at two other loci (ACO1, ZIP; see Olsen et al. 2007). Whereas the CYP79D15 presence/absence polymorphism again showed a perfect correlation with the Ac/ac phenotype, these polymorphisms were uncorrelated with genetic variation at either ACO1 (χ2 = 0.77, P > 0.1) or ZIP (χ2 = 0.16, P > 0.1), suggesting that the CYP79D15 correlation with Ac/ac is not an artifact of genomewide linkage disequilibrium. Definitive proof that CYP79D15 accounts for the Ac/ac polymorphism would be provided by genetic transformations of ac plants with CYP79D15 to generate the Ac phenotype.
White clover cyanogenesis and gene presence/absence polymorphisms:
The apparent molecular genetic basis of the Ac/ac polymorphism mirrors our findings for the Li/li polymorphism, which also arises through the presence/absence of the underlying gene (Olsen et al. 2007). Adaptive presence/absence polymorphisms have been documented for several other genes that function in plant defense (Stahl et al. 1999; Tian et al. 2002; Shen et al. 2006; reviewed by Tiffin and Moeller 2006). To our knowledge, all such previously documented cases have occurred in the model plant A. thaliana, and all have involved R genes, which function in pathogen recognition and initiation of the defense response.
For white clover, the finding that both the Ac/ac and the Li/li polymorphisms apparently arise through gene presence/absence polymorphisms suggests that the cyanogenesis polymorphism may derive from the allotetraploid origin of the species. White clover is thought to have originated through the hybridization of a cyanogenic species (contributing Ac and Li) and an acyanogenic species (possessing neither gene) (Williams and Williamson 2001; Badr et al. 2002). One of the hallmarks of allopolyploidization is the rapid and widespread elimination of genomic sequences in the immediate aftermath of the hybridization event (e.g., Feldman et al. 1997; Ozkan et al. 2001; reviewed by Ma and Gustafson 2005). Thus, if the cyanogenesis loci were deleted from the genomes of some plants and maintained in others following the hybridization event, and if selection acted to maintain these gene presence/absence polymorphisms as the genome became diploidized, then the Ac/ac and Li/li polymorphisms could date to the origin of the species.
An alternative hypothesis is that the Ac/ac and Li/li polymorphisms were already present in the cyanogenic diploid progenitor of white clover and that allotetraploidization occurred multiple times such that these polymorphisms were carried into white clover. The diploid species T. nigrescens has been reported to be polymorphic for the presence/absence of both cyanogenic glucosides and linamarase (Williams and Williamson 2001). T. nigrescens has also traditionally been considered a strong candidate to be a diploid progenitor of white clover, as it is one of few closely related cyanogenic species (Williams and Williamson 2001). Thus, the white clover cyanogenesis polymorphisms might represent ancient polymorphisms that predate the origin of the species.
Under both of these scenarios, the gene presence/absence polymorphisms are expected to represent longstanding balanced polymorphisms in white clover. This leads to the prediction that the genomic regions immediately flanking the cyanogenesis loci should bear molecular signatures of balancing selection. Such signatures have been detected for genomic regions flanking R gene presence/absence polymorphisms in A. thaliana (e.g., Stahl et al. 1999; Tian et al. 2002). Testing this hypothesis in white clover must await the identification of the flanking genomic sequences, as the genomic locations of Ac and Li are not yet known.
Molecular evolution of CYP79D15 sequences:
Our analyses of nucleotide variation at CYP79D15 do not reveal deviations from neutral equilibrium expectations. Because CYP79D15 sequences correspond to only one-half of the Ac/ac balanced polymorphism, analysis of these sequences would not necessarily be expected to reveal a signature of balancing selection. Analyses of Li sequences have similarly revealed no signature of balancing selection on the functional allele class (Olsen et al. 2007). Li sequences differ from CYP79D15, however, in that they show evidence of a selective sweep following an episode of positive directional selection (Olsen et al. 2007).
While CYP79D15 sequences do not show statistically significant deviations from neutrality, they do show a high level of protein-coding variation, with nearly double the number of nonsynonymous substitutions compared to synonymous substitutions, and a four- to five-codon indel polymorphism (Table 2). Some of this variation may have functional significance. Quantitative variation in cyanogenic glucosides among Ac plants has been commonly reported in white clover (e.g., Hughes 1991; see also materials and methods), and segregation analyses have indicated that a portion of this variation is attributable to differences among functional Ac alleles (Corkill 1942; Hughes et al. 1984). This suggests that molecular variation at CYP79D15 (or closely linked to it) may affect quantitative variation in cyanogenic glucosides. In this study we did not observe any obvious correlation between CYP79D15 haplotypes and cyanogenic glucoside levels (K. Olsen, unpublished observation). However, association studies following the identification of the CYP79D15 promoter may shed further light on the role of this gene in quantitative variation in cyanogenic glucosides.
Conclusions:
A major goal of modern evolutionary biology is to understand the molecular underpinnings of adaptation. While genetic model organisms such as A. thaliana and crop species are well suited to molecular evolutionary analysis, they are not necessarily ideal for understanding the ecological relevance of natural genetic variation. As an alternative approach, in this study we have examined an ecological model system: the ecology of white clover cyanogenesis has been studied for nearly a century (e.g., Armstrong et al. 1913). Here we have identified the gene that is likely to underlie the Ac/ac cyanogenic glucoside polymorphism; we have found that, like Li/li, this component of the cyanogenesis polymorphism apparently arises through a gene presence/absence polymorphism. With recent advances in the development of genetic and genomic tools for white clover (e.g., Barrett et al. 2004; Cogan et al. 2007), this species may prove a useful resource for further studies on the molecular genetics of adaptation.
Acknowledgments
The authors thank Pierre-François Perroud for technical advice on Southern hybridizations, Faith Steffen for assistance in SNP genotyping, other members of the Olsen lab group for helpful discussions and comments on the manuscript, and Washington University, which provided funds for this research to K. Olsen.
References
- Andersen, M. D., P. K. Busk, I. Svendsen and B. L. Møller, 2000. Cytochromes P-450 from cassava (Manihot esculenta Crantz) catalyzing the first steps in the biosynthesis of the cyanogenic glucosides linamarin and lotaustralin. J. Biol. Chem. 275 1966–1975. [DOI] [PubMed] [Google Scholar]
- Armstrong, H. E., E. F. Armstrong and E. Horton, 1913. Herbage studies. II.Variation in Lotus corniculatus and Trifolium repens: (cyanophoric plants). Proc. R. Soc. Lond. Ser. B 86 262–269. [Google Scholar]
- Badr, A., H. Sayed-Ahmed, A. El-Shanshouri and L. E. Watson, 2002. Ancestors of white clover (Trifolium repens L.), as revealed by isozyme polymorphisms. Theor. Appl. Genet. 106 143–148. [DOI] [PubMed] [Google Scholar]
- Bak, S., C. E. Olsen, B. L. Petersen, B. L. Møller and B. A. Halkier, 1999. Metabolic engineering of p-hydroxybenzylglucosinolate in Arabidopsis by expression of the cyanogenic CYP79A1 from Sorghum bicolor. Plant J. 20 663–671. [DOI] [PubMed] [Google Scholar]
- Bak, S., C. E. Olsen, B. A. Halkier and B. L. Møller, 2000. Transgenic tobacco and Arabidopsis plants expressing the two multifunctional Sorghum cytochrome P450 enzymes, CYP79A1 and CYP71E1, are cyanogenic and accumulate metabolites derived from intermediates in dhurrin biosynthesis. Plant Physiol. 123 1437–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak, S., S. M. Paquette, M. Morant, A. V. Morant, S. Saito et al., 2006. Cyanogenic glycosides: a case study for evolution and application of cytochromes P450. Phytochem. Rev. 5 309–329. [Google Scholar]
- Barrett, B., A. Griffiths, M. Schreiber, N. Ellison, C. Mercer et al., 2004. A microsatellite map of white clover. Theor. Appl. Genet. 109 596–608. [DOI] [PubMed] [Google Scholar]
- Barrett, T., C. G. Suresh, S. P. Tolley, E. J. Dodson and M. A. Hughes, 1995. The crystal structure of a cyanogenic beta-glucosidase from white clover, a family 1 glycosyl hydrolase. Structure 3 951–960. [DOI] [PubMed] [Google Scholar]
- Butler, G. W., and B. G. Butler, 1960. Biosynthesis of linamarin and lotaustralin in white clover. Nature 187 780–781. [Google Scholar]
- Cogan, N. O. I., M. C. Drayton, R. C. Ponting, A. C. Vecchies, N. R. Bannan et al., 2007. Validation of in silico-predicted genic SNPs in white clover (Trifolium repens L.), an outbreeding allopolyploid species. Mol. Genet. Genomics 277 413–425. [DOI] [PubMed] [Google Scholar]
- Collinge, D. B., and M. A. Hughes, 1982. a In vitro characterisation of the Ac locus in white clover (Trifolium repens L.). Arch. Biochem. Biophys. 218 38–45. [DOI] [PubMed] [Google Scholar]
- Collinge, D. B., and M. A. Hughes, 1982. b Developmental and physiological studies on the cyanogenic glucosides of white clover, Trifolium repens L. J. Exp. Bot. 33 154–161. [Google Scholar]
- Collinge, D. B., and M. A. Hughes, 1984. Evidence that linamarin and lotaustralin, the two cyanogenic glucosides of Trifolium repens L., are synthesised by a single set of microsomal enzymes controlled by the Ac/ac locus. Plant Sci. Lett. 34 119–125. [Google Scholar]
- Coop, I. E., 1940. Cyanogenesis in white clover III. Study of linamarase. NZ J. Sci. Technol. B 22–23 71–83. [Google Scholar]
- Corkill, L., 1942. Cyanogenesis in white clover (Trifolium repens). V. The inheritance of cyanogenesis. NZ J. Sci. Technol. B 23 178–193. [Google Scholar]
- Daday, H., 1954. a Gene frequencies in wild populations of Trifolium repens. I. Distribution by latitude. Heredity 8 61–78. [Google Scholar]
- Daday, H., 1954. b Gene frequencies in wild populations of Trifolium repens. II. Distribution by altitude. Heredity 8 377–384. [Google Scholar]
- Daday, H., 1958. Gene frequencies in wild populations of Trifolium repens. III. World distribution. Heredity 12 169–184. [Google Scholar]
- Daday, H., 1965. Gene frequencies in wild populations of Trifolium repens. IV. Mechanisms of natural selection. Heredity 20 355–365. [Google Scholar]
- De Araujo, A. M., 1976. The relationship between altitude and cyanogenesis in white clover (Trifolium repens L.). Heredity 37 291–293. [Google Scholar]
- Dirzo, R., and J. L. Harper, 1982. a Experimental studies on slug–plant interactions. III. Differences in the acceptability of individual plants of Trifolium repens to slugs and snails. J. Ecol. 70 101–117. [Google Scholar]
- Dirzo, R., and J. L. Harper, 1982. b Experimental studies on slug–plant interactions. IV. The performance of cyanogenic and acyanogenic morphs of Trifolium repens in the field. J. Ecol. 70 119–138. [Google Scholar]
- Dirzo, R., and J. Sarukhan, 1984. Perspectives on Plant Population Ecology. Sinauer Associates, Sunderland, MA.
- Ennos, R. A., 1981. Detection of selection in populations of white clover (Trifolium repens L.). Biol. J. Linn. Soc. 15 75–82. [Google Scholar]
- Fay, J. C., and C.-I. Wu, 2000. Hitchhiking under positive Darwinian selection. Genetics 155 1405–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigl, F., and V. Anger, 1966. Replacement of benzidine by copper ethylacetoacetate and tetra base as spot-test reagent for hydrogen cyanide and cyanogen. Analyst 91 282–284. [DOI] [PubMed] [Google Scholar]
- Feldman, M., B. Liu, G. Segal, S. Abbo, A. A. Levy et al., 1997. Rapid elimination of low-copy DNA sequences in polyploid wheat: a possible mechanism for differentiation of homoeologous chromosomes. Genetics 147 1381–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forslund, K., M. Morant, B. Jorgensen, C. E. Olsen, E. Asamizu et al., 2004. Biosynthesis of the nitrile glucosides rhodiocyanoside A and D and the cyanogenic glucosides lotaustralin and linamarin in Lotus japonicus. Plant Physiol. 135 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds, W., and J. P. Grime, 1972. The response of cyanogenic and acyanogenic phenotypes of Trifolium repens to soil moisture supply. Heredity 28 181–187. [Google Scholar]
- Ganders, F. R., 1990. Altitudinal clines for cyanogenesis in introduced populations of white clover near Vancouver, Canada. Heredity 64 387–390. [Google Scholar]
- Hall, T., 2001. Biolign alignment and multiple contig editor. http://en.bio-soft.net/dna/BioLign.html.
- Hayden, K. J., and I. M. Parker, 2002. Plasticity in cyanogenesis of Trifolium repens L.: inducibility, fitness costs and variable expression. Evol. Ecol. Res. 4 155–168. [Google Scholar]
- Hughes, M. A., 1991. The cyanogenic polymorphism in Trifolium repens L. (white clover). Heredity 66 105–115. [Google Scholar]
- Hughes, M. A., and E. E. Conn, 1976. Cyanoglucoside biosynthesis in white clover (Trifolium repens L.). Phytochemistry 15 697–701. [Google Scholar]
- Hughes, M. A., J. D. Stirling and D. B. Collinge, 1984. The inheritance of cyanoglucoside content in Trifolium repens L. Biochem. Genet. 22 139–151. [DOI] [PubMed] [Google Scholar]
- Kakes, P., 1989. An analysis of the costs and benefits of the cyanogenic system in Trifolium repens L. Theor. Appl. Genet. 77 111–118. [DOI] [PubMed] [Google Scholar]
- Kristensen, C., M. Morant, C. E. Olsen, C. T. Ekstrom, D. W. Galbraith et al., 2005. Metabolic engineering of dhurrin in transgenic Arabidopsis plants with marginal inadvertent effects on the metabolome and transcriptome. Proc. Natl. Acad. Sci. USA 102 1779–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, X.-F., and J. P. Gustafson, 2005. Genome evolution of allopolyploids: a process of cytological and genetic diploidization. Cytogenet. Genome Res. 109 236–249. [DOI] [PubMed] [Google Scholar]
- Majumdar, S., K. K. De and S. Banerjee, 2004. Influence of two selective forces on cyanogenesis polymorphism of Trifolium repens L. in Darjeeling Himalaya. J. Plant Biol. 47 124–128. [Google Scholar]
- McDonald, J. H., and M. Kreitman, 1991. Adaptive protein evolution at the Adh locus in Drosophila. Nature 351 652–654. [DOI] [PubMed] [Google Scholar]
- Melville, J., and B. W. Doak, 1940. Cyanogenesis in white clover II. Isolation of glucoside constituents. NZ J. Sci. Technol. B 22 67–70. [Google Scholar]
- Nei, M., 1987. Molecular Evolutionary Genetics. Columbia University Press, New York.
- Nelson, D. R., 2006. Cytochrome P450 nomenclature, 2004, pp. 1–10 in Cytochrome P450 Protocols, Ed. 2 (Methods in Molecular Biology, Vol. 320), edited by I. R. Phillips and E. A. Shephard. Humana Press, Totowa, NJ. [DOI] [PubMed]
- Olsen, K. M., B. L. Sutherland and L. L. Small, 2007. Molecular evolution of the Li/li chemical defence polymorphism in white clover (Trifolium repens L.). Mol. Ecol. 16 4180–4193. [DOI] [PubMed] [Google Scholar]
- Oxtoby, E., M. A. Dunn, A. Pancoro and M. A. Hughes, 1991. Nucleotide and derived amino acid sequence of the cyanogenic beta-glucosidase (linamarase) from white clover (Trifolium repens L.). Plant Mol. Biol. 17 209–219. [DOI] [PubMed] [Google Scholar]
- Ozkan, H., A. A. Levy and M. Feldman, 2001. Allopolyploidy-induced rapid genome evolution in the wheat (Aegilops-Triticum) group. Plant Cell 13 1735–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson, G. A., and G. E. Brink, 1998. Cyanogenesis effect on insect damage to seedling white clover in a bermudagrass sod. Agron. J. 90 208–210. [Google Scholar]
- Porebski, S. L., G. Bailey and B. R. Baum, 1997. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Rep. 15 8–15. [Google Scholar]
- Rozas, J., J. C. Sánchez-DelBarrio, X. Messeguer and R. Rozas, 2003. DNAsp, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19 2496–2497. [DOI] [PubMed] [Google Scholar]
- Saucy, F., J. Studer, V. Aerni and B. Schneiter, 1999. Preference for acyanogenic white clover (Trifolium repens) in the vole Arvicola terrestris: I. Experiments with two varieties. J. Chem. Ecol. 25 1441–1454. [Google Scholar]
- Seigler, D. S., and A. M. Brinker, 1993. Characterisation of cyanogenic glucosides, cyanolipids, nitroglycosides, organic nitro compounds and nitrile glucosides from plants. Methods Plant Biochem. 8 51–131. [Google Scholar]
- Shen, J. D., H. Araki, L. L. Chen, J. Q. Chen and D. C. Tian, 2006. Unique evolutionary mechanism in R-genes under the presence/absence polymorphism in Arabidopsis thaliana. Genetics 172 1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvertown, J., and D. Charlesworth, 2001. Introduction to Plant Population Biology, Ed. 4. Blackwell Science, Oxford.
- Stahl, E. A., G. Dwyer, R. Mauricio, M. Kreitman and J. Bergelson, 1999. Dynamics of disease resistance polymorphism at the Rpm1 locus of Arabidopsis. Nature 400 667–671. [DOI] [PubMed] [Google Scholar]
- Tajima, F., 1989. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, D. C., H. Araki, E. Stahl, J. Bergelson and M. Kreitman, 2002. Signature of balancing selection in Arabidopsis. Proc. Natl. Acad. Sci. USA 99 11525–11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffin, P., and D. A. Moeller, 2006. Molecular evolution of plant immune system genes. Trends Genet. 22 662–670. [DOI] [PubMed] [Google Scholar]
- Till-Bottraud, I., P. Kakes and B. Dommee, 1988. Variable phenotypes and stable distribution of the cyanotypes of Trifolium repens L. in southern France. Acta Oecol. 9 393–404. [Google Scholar]
- Viette, M., C. Tettamanti and F. Saucy, 2000. Preference for acyanogenic white clover (Trifolium repens) in the vole Arvicola terrestris. II. Generalization and further investigations. J. Chem. Ecol. 26 101–122. [Google Scholar]
- Ware, W. M., 1925. Experiments and observations on forms and strains of Trifolium repens. J. Agric. Sci. 15 47–67. [Google Scholar]
- Watterson, G. A., 1975. On the number of segregating sites in genetical models without recombination. Theor. Popul. Biol. 7 256–276. [DOI] [PubMed] [Google Scholar]
- Williams, W. M., and M. L. Williamson, 2001. Genetic polymorphism for cyanogenesis and linkage at the linamarase locus in Trifolium nigrescens Viv. subsp. nigrescens. Theor. Appl. Genet. 103 1211–1215. [Google Scholar]