Abstract
Shoot apical meristem (SAM) development is coordinately regulated by two interdependent signaling events: one maintaining stem cell identity and the other governing the initiation of lateral organs from the flanks of the SAM. The signaling networks involved in this process are interconnected and are regulated by multiple molecular mechanisms. Class III homeodomain-leucine zipper (HD-ZIP III) proteins are the most extensively studied transcription factors involved in this regulation. However, how different signals are integrated to maintain stem cell identity and to pattern lateral organ polarity remains unclear. Here, we demonstrated that a small ZIP protein, ZPR3, and its functionally redundant homolog, ZPR4, negatively regulate the HD-ZIP III activity in SAM development. ZPR3 directly interacts with PHABULOSA (PHB) and other HD-ZIP III proteins via the ZIP motifs and forms nonfunctional heterodimers. Accordingly, a double mutant, zpr3-2 zpr4-2, exhibits an altered SAM activity with abnormal stem cell maintenance. However, the mutant displays normal patterning of leaf polarity. In addition, we show that PHB positively regulates ZPR3 expression. We therefore propose that HD-ZIP III activity in regulating SAM development is modulated by, among other things, a feedback loop involving the competitive inhibitors ZPR3 and ZPR4.
INTRODUCTION
Shoot apical meristem (SAM) development is regulated by coordinate interactions of two major groups of interdependent signaling events, in which one group of signals maintaining stem cell identity is balanced with the other group of signals governing the initiation of lateral organs from the flanks of the SAM (Williams and Fletcher, 2005; Byrne, 2006). Accumulating evidence also supports that signals from the adaxial domains of lateral organs are important for SAM formation (Eshed et al., 2001). Several key regulators, including class III homeodomain-leucine zipper (HD-ZIP III) transcription factors (Emery et al., 2003; Bowman, 2004; Green et al., 2005; Kim et al., 2005; Prigge et al., 2005; Williams et al., 2005), a group of KNOX and KANADI (KAN) proteins, and miR165/166 (Juarez et al., 2004; Vaucheret et al., 2004), mediate the signaling crosstalk (Kerstetter et al., 2001). Recently, a trans-acting small interfering RNA has been shown to restrict the accumulation domains of miR165/166 and HD-ZIP III mRNAs (Nogueira et al., 2007), providing an additional level of signaling complexity.
The SAM exhibits a distinct cellular morphology, in which a population of slowly dividing stem cells is located in the central zone (CZ). Cells in the CZ are either used to maintain the integrity of the stem cell population or are displaced to the peripheral or rib zones (Carraro et al., 2006; Golz, 2006). A small group of cells in the peripheral zone then are switched developmentally from an indeterminate to determinate fate to initiate lateral organ formation.
A series of surgical experiments has shown that a polarizing signal arising in the meristem promotes the adaxial identity in the cells closer to the meristem and the abaxial identity of those located away from the meristem (Reinhardt et al., 2005; Byrne, 2006). Signals from the adaxial domains of leaf primordia also are essential for stem cell maintenance. Overexpression of an abaxial promoting KAN gene in the adaxial domain of cotyledons results in the loss of embryonic SAM and abaxialization of leaves (Eshed et al., 2001). By contrast, a dominant phabulosa (phb) mutant, in which an adaxial promoting PHB gene is ectopically expressed, has an enlarged SAM (McConnell and Barton, 1998).
One potential candidate for the polarizing signal is miR165/166. It is expressed predominantly in the abaxial domain of lateral organs (Juarez et al., 2004; Kidner and Martienssen, 2004). Notably, maize (Zea mays) miR166 originally is expressed basally in the developing leaf primordium and subsequently accumulates in the abaxial domain of the growing organ, possibly by migrating through the phloem (Juarez et al., 2004). However, the view of miR165/166 being a polarizing signal is not supported by the expression patterns of the HD-ZIP III genes. Each HD-ZIP III gene exhibits a distinct expression domain, and the promoter activity of each matches its mRNA distribution (Baima et al., 1995; McConnell et al., 2001; Otsuga et al., 2001; Golz, 2006). In addition, it has been shown that miR165/166 is expressed throughout the leaf primordia (Li et al., 2005). However, PHB mRNA level also is elevated both in the abaxial and adaxial domains of lateral organs in phb-1d (McConnell et al., 2001).
A newly elucidated way of controlling transcription factors is via non-DNA binding proteins (Klemm et al., 1998). For example, the inhibitor of DNA binding protein (ID) in animals has a basic helix-loop-helix (bHLH) motif but lacks the basic DNA binding region (Benezra et al., 1990). It associates with several bHLH transcription factors, such as MyoD, E12, and E47, and represses their activities in activating transcription by forming nonfunctional heterodimers. Recently, the Arabidopsis thaliana KIDARI protein, which has a structural organization similar to ID, has been suggested to exert its role by physically interacting with a bHLH transcription factor, HFR1, involved in photomorphogenesis (Hyun and Lee, 2006).
In this work, we demonstrate that a small ZIP protein, ZPR3, is a competitive inhibitor of the HD-ZIP III transcription factors in SAM development. ZPR3 represses the activities of the HD-ZIP III members in activating transcription by forming nonfunctional heterodimers via the ZIP motifs. An activation-tagged mutant, zpr3-1d, exhibits a disrupted SAM activity and partially abaxialized leaf, as has been observed in the phb phv rev triple mutant (Emery et al., 2003). By contrast, the zpr3-2 zpr4-2 knockout mutant has a disorganized SAM activity, but its polar patterning of lateral organs is essentially normal. Interestingly, PHB positively regulates the ZPR3 expression. Our findings demonstrate that the HD-ZIP activities in SAM development are regulated via competitive inhibition.
RESULTS
The zpr3-1d Mutant Lacks a Functional SAM
We isolated a morphogenic mutant with notably reduced growth and narrow leaves (Figure 1A), designated zpr3-1d, from an Arabidopsis activation-tagged mutant pool generated by randomly integrating the cauliflower mosaic virus (CaMV) 35S enhancer into the genome of ecotype Columbia (Col-0) (Weigel et al., 2000). The zpr3-1d leaves were also severely curled downward (see Supplemental Figure 1A online). Close examination of the zpr3-1d leaf surfaces by scanning electron microscopy revealed that the adaxial surface was irregular like the abaxial surface (Figure 1B). However, the polarity of the leaves was apparently unchanged in the mutant (see Supplemental Figure 1B online), indicating that the zpr3-1d leaves were partially abaxialized. Consistent with this, the expression levels of YAB3, KAN1, and KAN2, which promote the abaxial identity, were elevated by approximately twofold in the mutant (Figure 1C). By contrast, transcript levels of the HD-ZIP III genes sustaining the adaxial identity were not altered to a discernible level, except for ATHB15 (Figure 1D). The ATHB15 transcript level was elevated by ∼60%. Although it was not further examined in this work, the vascular structure may be affected in the mutant (Kim et al., 2005).
Figure 1.
Disrupted SAM and Partially Abaxialized Leaves in zpr3-1d.
(A) Phenotypes of zpr3-1d and zpr3-2 compared with the wild type (Col-0). A full-size ZPR3 cDNA was overexpressed under the control of the CaMV 35S promoter to confirm the zpr3-1d phenotype (35S:ZPR3). Five-week-old plants were photographed.
(B) Partial abaxialization of the zpr3-1d leaves. The third rosette leaves harvested 10 d after stratification were examined by scanning electron microscopy. Note that the adaxial epidermal cells of the mutant have irregular shapes like the abaxial epidermal cells of the wild type. Bar = 50 μm.
(C) Upregulation of some genes promoting abaxial cell identity in zpr3-1d. Transcript levels were measured by real-time RT-PCR using the aerial parts of 10-d-old plants grown on Murashige and Skoog (MS)-agar plates. Means + se are shown (n = 3).
(D) Transcript levels of the HD-ZIP III genes in zpr3-1d. Transcript levels were measured by real-time RT-PCR using the aerial parts of 10-d-old plants grown on MS-agar plates. Means + se are shown (n = 3).
(E) Absence of secondary shoots in zpr3-1d. Inset shows an enlarged view of a zpr3-1d cauline leaf axil. Note bare axil, indicative of no lateral meristem (arrow).
(F) Schematic of the T-DNA insertion sites in the genomes of zpr3-1d (white triangle) and zpr3-2 (black triangle). Chr 3, chromosome 3.
(G) Phenotypes of the 35S:ZPR3 transgenic plants. Two major types of the apices observed in 10-d-old seedlings are shown: one with a pin-like structure and the other without a SAM (left panel), longitudinal sections of which are shown in the right top and bottom panels, respectively.
(H) Transgenic plants overexpressing ZPR3 under the control of an estradiol-inducible promoter. Only newly emerging leaves were curled downward after estradiol induction (20 μM) 22 d after stratification (bottom panel). Transcript levels were measured by RT-PCR. A tubulin gene (TUB) was used as control for constitutive expression.
zpr3-1d produced fewer secondary shoots (Figure 1E; see Supplemental Figure 2 online). Notably, some zpr3-1d lines had trumpet-shaped cotyledons and an absent or severely arrested SAM with a pin-like structure at the apex (see Supplemental Figure 3 online). The cauline leaf axils were mostly bare without lateral meristems (Figure 1E). This phenotype is quite similar to that observed in the revoluta (rev) mutant (Talbert et al., 1995), suggesting a role for ZPR3 in SAM development and lateral organ patterning.
ZPR3 Encodes a Small ZIP Protein
Three-step thermal asymmetric interlaced PCR (Liu et al., 1995) was employed to map the T-DNA insertion site in the zpr3-1d genome. The result showed that a single copy of the 35S enhancer was inserted into the region between At3g52760 and At3g52770 (Figure 1F). In the mutant, the At3g52770 gene was drastically activated (see Supplemental Figure 4 online), while the At3g52760 gene was not. DNA gel blot hybridization confirmed that zpr3-1d contained a single copy of the T-DNA insertion (data not shown). In addition, all BASTA-resistant progeny of the zpr3-1d plants exhibited the zpr3-1d phenotype, indicating that the zpr3-1d mutation cosegregates with the T-DNA insertion.
The At3g52770 gene encodes a small protein consisting of 67 residues (see Supplemental Figure 5 online). Database searches revealed that the encoded protein is LITTLE ZIPPER3 (ZPR3), a member of a small group of leucine zipper–containing proteins consisting of ZPR1 to ZPR4 (Wenkel et al., 2007). ZPR3 possesses residue identities of 37, 38, and 75% with ZPR1, ZPR2, and ZPR4, respectively (see Supplemental Figure 5 online).
Overexpression of ZPR3 under the control of the CaMV 35S promoter (35S:ZPR3) recapitulated the zpr3-1d phenotype (Figures 1A and 1G). Most of the transgenic plants obtained (>100 transgenic plants) exhibited reduced growth. Approximately 40% of them had downward leaf curling, and ∼30% of them had no or severely arrested meristems (see Supplemental Figure 6 online), demonstrating that ZPR3 activation underlies the zpr3-1d phenotypes. By contrast, the primary root growth of 35S:ZPR3 and zpr3-1d was essentially normal, indicating that ZPR3 regulates specifically SAM development.
To confirm the correlation between ZPR3 and the zpr3-1d phenotype, an estradiol-inducible promoter was used to direct ZPR3 expression (Zuo et al., 2000). When the transgenic plants were treated with estradiol, only the newly emerging leaves were curled downward (Figure 1H), suggesting that ZPR3 functions at the early stage of leaf development.
ZPR3 Interacts with HD-ZIP III Proteins via the ZIP Motifs
The central region of ZPR3 contains a ZIP motif (see Supplemental Figure 5 online) that is most similar to those present in the HD-ZIP III proteins. However, ZPR3 lacks the region of those proteins that mediates DNA binding.
Based on the structural similarity between ZPR3 and HD-ZIP IIIs, we predicted that they might physically interact with one another. Yeast two-hybrid assays were employed to examine the hypothesis. ATHB2, which is a HD-ZIP II member (Ruberti et al., 1991), was included as a negative control in the assays. ZPR3 indeed interacted with PHB, PHV, ATHB15, and ATHB8 (see Supplemental Figure 7 online). By contrast, it did not interact with ATHB2 or REV. To confirm the results, in vitro pull-down assays were employed. A recombinant glutathione S-transferase (GST)-ZPR3 fusion and the HD-ZIP III proteins synthesized by in vitro translation in the presence of 35S-Met were used. All five HD-ZIP III members interacted with ZPR3 (Figure 2A), whereas ATHB2 did not (see Supplemental Figure 8 online). Notably, REV, which did not show any interactions with ZPR3 in yeast cells, also interacted with ZPR3. Our observations are in agreement with previous work (Wenkel et al., 2007) demonstrating that the ZPR proteins interact with multiple HD-ZIP III members.
Figure 2.
Interactions of ZPR3 with HD-ZIP III Proteins via the ZIP Motifs.
(A) In vitro pull-down assays. A recombinant GST-ZPR3 fusion or GST alone was incubated with in vitro–translated, radiolabeled HD-ZIP III proteins. Bound HD-ZIP III proteins were separated on a 12% SDS-PAGE and subjected to autoradiography.
(B) Mapping of the interacting domain in PHB. The numbers denote residue positions for each construct (schematics are shown in the left panel). Interactions were examined both by growth of yeast cells in selective media (-QD) and by in vitro pull-down assays (right panel). IN, input; Y2H, yeast two-hybrid assays; HD, homeodomain; ZIP, leucine zipper; START, StAR-related lipid transfer; AA, amino acids.
(C) Interactions of ZPR3 with PHB and REV. Total cellular extracts from N. benthamiana cells expressing MYC fusions of PHB (PH), REV (RE), and ATHB2 (HB) were incubated with GST or GST-ZPR3. Protein gel blots of interacting proteins probed with an anti-MYC antibody are shown. The amounts of the MYC fusion proteins were immunologically normalized using the same antibody before use (Input). Coomassie blue–stained gels are displayed at the bottom to show the amounts of GST and GST-ZPR3 used.
(D) Subcellular localization of ZPR3. A ZPR3-GFP fusion was transiently expressed in onion epidermal cells.
We next examined which structural motif in the HD-ZIP III proteins was responsible for the ZPR3–HD-ZIP III interactions. We generated a series of PHB deletions and examined their interactions with ZPR3 both by yeast coexpression and in vitro pull-down assays. All the deletion constructs containing the ZIP motif efficiently interacted with ZPR3, but those without the ZIP motif did not (Figure 2B), indicating that the ZIP motif was necessary and sufficient for the interactions. The ZPR3-PHB interactions were further examined using the Nicotiana benthamiana transient expression system (Llave et al., 2002). The miR166/165 binding sequences of the PHB and REV genes were mutated to optimize protein production and transiently expressed in N. benthamiana cells (Kim et al., 2005). Total cellular extracts were then probed with the GST-ZPR3 fusion. Again, ZPR3 interacted with PHB and REV but not with ATHB2 (Figure 2C).
When a ZPR3-GFP fusion was transiently expressed in onion epidermal cells, the green fluorescent protein (GFP) signal was predominantly detected in the nucleus (Figure 2D), consistent with the ZPR3–HD-ZIP III interactions occurring there.
ZPR3 Inhibits HD-ZIP III Dimerization
PHV, and possibly other HD-ZIP III proteins, binds to DNA as a homodimer (Sessa et al., 1998). Our data indicated that ZPR3 binds to the ZIP motif of HD-ZIP III proteins and negatively regulates their activities. It was therefore envisioned that ZPR3 might block the homodimer formation of the HD-ZIP III proteins. To examine the effects of ZPR3 on PHB dimerization, ZPR3 was expressed under the control of a Met-suppressible promoter, pMET25, in yeast cells (MET25:ZPR3; Figure 3A) (Olesen et al., 2000). The PHB gene was fused in frame to a gene construct encoding the activation domain (AD) of GAL4, and the AD-PHB fusion was coexpressed with the MET25:ZPR3 construct. A truncated PHB gene encoding the N-terminal half of PHB (residues 1 to 174) was fused in frame to the binding domain (BD) coding sequence of GAL4, and the BD-ΔC construct was cotransformed into the yeast cells.
Figure 3.
Inhibition of Dimerization and Activity of PHB by ZPR3.
(A) PHB dimerization assays by yeast coexpression. ZPR3 was expressed under the control of the Met-suppressible promoter (pMET25) (MET25:ZPR3). The ZPR3 gene is not expressed on selective media without Leu, Trp, and His (-LWH) but is expressed on selective media without Leu, Trp, His, and Met (-LWHM). Cell growth on the -LW media indicates that the yeast cells contain the two plasmid DNAs. The ΔC construct of PHB includes residues 1 to 174.
(B) PHB dimerization and activity assays by β-galactosidase (β-Gal) activity measurements. For the measurements of activities in transcription-activation, a BD-PHB fusion construct containing a full-size PHB protein was used. The β-Gal activities were normalized by dividing total activity by optical cell density. Means + se are shown (n = 3).
(C) Effects of ZPR3 on PHB protein stability. An anti-MYC antibody was used to immunologically measure the levels of the MYC-PHB proteins in transgenic plants coexpressing a MYC-PHB fusion under the CaMV 35S promoter and ZPR3 under the estradiol-inducible promoter. The Coomassie blue–stained gel is displayed at the bottom.
(D) Effects of ZPR3 on PHB transcription. Transcript levels in the plants from (C) were measured by RT-PCR. A tubulin gene (TUB) was used as control for constitutive expression.
(E) A genetic cross between phb-1d-Col and zpr3-1d. The inset shows an enlarged view of the phb-1d-Col plant. Four-week-old plants were photographed.
(F) Comparison of the inflorescences of the parental mutants and the zpr3-1d phb-1d-Col double mutant. ZPR3 transcript levels were measured by RT-PCR (bottom panel). A tubulin gene (TUB) was used as control for constitutive expression.
(G) Comparison of flowers and leaves of the parental mutants and the zpr3-1d phb-1d-Col double mutant. The fifth leaves were photographed.
(H) A genetic cross between zpr3-1d and transgenic plants overexpressing REV (35S:REV). Three-week-old plants were photographed.
AD-PHB efficiently interacted with BD-ΔC in the absence of ZPR3 induction, as examined by yeast coexpression (Figure 3A) and by β-galactosidase activity assays (Figure 3B). By contrast, ZPR3 induction substantially repressed the interaction, demonstrating that ZPR3 blocks the PHB dimerization.
We next examined whether the transcription-activation activity of PHB is influenced by ZPR3. A full-size PHB gene was fused in frame with the BD-coding sequence of GAL4, and the BD-PHB fusion was transformed into the yeast cells containing the MET25:ZPR3 construct. In the absence of ZPR3 induction, the BD-PHB protein was transcriptionally active (Figure 3B). However, ZPR3 induction drastically reduced its activity, strongly supporting that ZPR3 represses the activity of PHB in activating transcription. Wenkel et al. (2007) have shown that ZPR3 blocks binding of REV to DNA, like the ID proteins in animals (Benezra et al., 1990; Perk et al., 2005). It is therefore likely that ZPR3 represses the activity of PHB by forming nonfunctional heterodimers.
To rule out the possibility that ZPR3 affects the PHB protein stability, we generated transgenic plants coexpressing a MYC-PHB fusion, in which six copies of the MYC epitope tag-coding sequence were fused in frame to the PHB gene, under the CaMV 35S promoter, and ZPR3 under the estradiol-inducible promoter. Protein gel blot analysis using an anti-MYC antibody demonstrated that ZPR3 induction does not affect the PHB protein stability (Figure 3C). PHB transcription was also unaffected by ZPR3 induction (Figure 3D).
ZPR3 Inhibits PHB Activity in Planta
To explore the effects of ZPR3 on the PHB activity in planta, zpr3-1d plants were crossed with phb-1d plants, and the phenotypes were compared with those of the parental mutants. Since PHB activity is inhibited by ZPR3, it was predicted that the phenotypes of phb-1d or zpr3-1d would disappear in the phb-1d zpr3-1d double mutant. For the cross, phb-1d (in Landsberg erecta background) was first backcrossed to the Col-0 ecotype twice to obtain a phb-1d-Col line. The phb-1d-Col phenotype was essentially similar to the phb-1d phenotype (see Supplemental Figure 9 online). It developed lateral organs with radial symmetry and wavy inflorescence stems (Figures 3E and 3F).
The leaves and flowers of the phb-1d-Col zpr3-1d mutant were phenotypically similar to those of wild-type plants (Figure 3G). In addition, the double mutant generated more axillary shoots than zpr3-1d, and the inflorescences and inflorescence stems were essentially normal (Figure 3F). These observations indicate that ZPR3 overexpression suppresses at least in part the phb-1d-Col phenotype and that ZPR3 negatively regulates PHB in planta.
In addition, when zpr3-1d was crossed with transgenic plants overexpressing REV (35S:REV) (see Supplemental Figure 10 online), the downward leaf curling of zpr3-1d and the upward leaf curling of the 35S:REV plants were rescued (Figure 3H; see Supplemental Figure 10 online), suggesting that ZPR3 might target multiple HD-ZIP III proteins.
ZPR3 Is Essential for SAM Function
To obtain more insights into the molecular mechanism of ZPR3 action, we isolated a ZPR3 mutant (zpr3-2), in which a single copy of T-DNA was inserted into the 5′ untranslated region (Figure 4A).
Figure 4.
Phenotypes of zpr3-2 and zpr3-2 zpr4-2.
(A) T-DNA insertion site (arrow) in the zpr3-2 genome. The black box denotes a translating exon, and the white boxes denote nontranslating exons. Transcript levels were measured by RT-PCR (right panel). A tubulin gene (TUB) was used as control for constitutive expression.
(B) Phenotype of an adult zpr3-2 mutant compared with the wild type. Four-week-old plants were photographed.
(C) Inflorescences of zpr3-2 compared with the wild type. An enlarged view of a mutant node with three inflorescences (IN) is shown in the bottom panel.
(D) Short internodes of zpr3-2. Distribution of wild-type and mutant internode lengths.
(E) Phenotype of zpr3-2 zpr4-2 compared with the wild type at seedling (top) and adult (bottom) stages. The images were obtained by scanning electron microscopy. The mutant seedling has three cotyledons (top panel) 9 d after stratification. Note the clustering of cauline leaves due to short internodes (bottom panel). Bars =1 mm.
(F) Phenotype of zpr3-2 zpr4-2 compared with the wild type 27 d after stratification. Left top panel, control plant; the other three panels, the mutant plants.
(G) Phenotype of zpr3-2 zpr4-2 compared with the wild type 45 d after stratification.
(H) Phenotypic comparison of wild-type, phb-1d, and zpr3-2 zpr4-2 plants. Five-week-old plants were photographed.
The zpr3-2 mutant was phenotypically indistinguishable from control plants during the vegetative growth. However, visible phenotypic changes were apparent during reproductive growth. Although its height was similar to that of control plants (Figure 4B), the internodes between siliques were often absent or very short in ∼90% of the mutant plants (Figures 4B to 4D). In some plants, two or more secondary inflorescences developed at the cauline leaf axils (Figure 4C, top panel). In Arabidopsis, the vegetative SAM produces rosette leaves that are close together because of the lack of internode elongation. However, during the transition to reproductive growth, the internodes elongate and paraclade primordia are initiated on the cauline leaf axils. Our observations would therefore indicate that the activities of the SAM and/or axillary meristems are disrupted in zpr3-2. Transformation of zpr3-2 with a genomic DNA fragment containing the ZPR3 gene with the endogenous promoter completely rescued the zpr3-2 phenotype (see Supplemental Figure 11 online), confirming that the zpr3-2 phenotype is caused by ZPR3 inactivation.
ZPR4, consisting of 72 residues, is the most similar to ZPR3 in sequence and length among the ZPR proteins (see Supplemental Figure 5 online). It was therefore hypothesized that the absence of discernible phenotypes in the vegetative growth stage of zpr3-2 would be due to functional redundancy. To examine this, a zpr3-2 zpr4-2 double mutant was obtained by crossing zpr3-2 with zpr4-2 (see Supplemental Figure 12 online). The double mutant was verified by complementation with the ZPR3 gene with its own promoter before analysis (see Supplemental Figure 11 online).
Notably, distinct phenotypic alterations were observed in the zpr3-2 zpr4-2 mutant throughout the plant growth stages. The zpr3-2 zpr4-2 seedlings consistently had extra cotyledons, 35 out of 40 had three cotyledons while the remaining five had four cotyledons (Figure 4E, top panel). At later growth stages, the mutant produced three or four true leaves simultaneously between the cotyledons (Figure 4E, bottom panel), after which the mutant did not produce any more leaves. Interestingly, a few days later, the mutant resumed producing leaves at random positions (Figure 4F). The fully grown mutant produced >50 leaves before flowering, resulting in a bushy appearance (Figure 4G). Furthermore, phyllotaxy was also evidently altered in the mutant, similar to that in phb-1d (Figure 4H). Together, these observations indicate that leaf initiation and phyllotaxy is disturbed in the mutant, possibly because of aberrant SAM activity. Indeed, the overall shape and development of the zpr3-2 zpr4-2 SAM was quite distinct from that of control plants based on scanning electron microscopy observations. Although a SAM-like structure appeared at the apex of the mutant, its surface was irregular with several bulges (Figure 5A, DAS 9). In addition, individual mutant plants exhibited an array of altered SAM-like structures, including radially symmetric organs (Figure 5C).
Figure 5.
Altered SAM Morphologies and Flower and Leaf Phenotypes in zpr3-2 zpr4-2.
(A) SAM morphology of zpr3-2 zpr4-2. The left top panel shows the SAM of a wild-type control plant. The other panels show the SAM of the double mutant at different growth stages. Arrows indicate the developing leaf primordia. DAS, days after stratification. Bars = 50 μm.
(B) A vertical section of zpr3-2 zpr4-2 apex. Arrows indicate the leaf primordia that are ectopically produced. The SAM-like structure is observed at the center of this section. Bar = 50 μm.
(C) Various types of zpr3-2 zpr4-2 SAMs 18 d after stratification. Bars = 100 μm.
(D) Abnormal floral structure of zpr3-2 zpr4-2 compared with the wild type. In some flowers, multiple carpels were formed.
(E) Ovule-like appearance on the surface of zpr3-2 zpr4-2 ovary (arrow).
(F) Scanning electron micrograph showing papillae-like structures at the petal margin in zpr3-2 zpr4-2. These characteristic are indicative of homeotic transformation. Bar = 200 μm.
(G) Scanning electron micrograph of the inflorescence stem of zpr3-2 zpr4-2. It is fasciated (top panel), and ectopic leaves are produced on the surface (bottom panel). Bars = 500 μm.
In later developmental stages, a small bulge appeared at the center of the apex region (Figure 5A, DAS 16). Interestingly, more careful examination revealed that leaf primordia were not produced from the SAM-like structure. Instead, they originated from the adaxial side of leaf/cotyledon bases and randomly from the flat region around the SAM-like structure (Figure 5A, DAS 18 and later). Vertical sectioning of the zpr3-2 zpr4-2 apex confirmed the results (Figure 5B). The apex structure of the zpr3-2 zpr4-2 mutant explains why the mutant produces numerous leaves (Figure 4G) and why it has an altered phyllotaxy (Figure 4H). Collectively, these observations indicate that the zpr3-2 zpr4-2 SAM is nonfunctional, but many ectopic meristems develop around the SAM-like structure, further supporting that ZPR3 and ZPR4 are essential for proper functioning of stem cells in the SAM.
Numerous protrusions and rumples were observed around the SAM-like structure (Figure 5A, DAS 23 and 33), from which many floral primordia (as well as leaf primordia) initiated. These may be due to ectopic meristem activity and the disturbance of certain molecular mechanisms underlying the SAM development.
Our data indicated that ZPR3 negatively regulates PHB activity. It was therefore predicted that PHB activity would be enhanced in the zpr3-2 zpr4-2 mutant. If this were the case, the inactivation of PHB would reduce the phenotypes of the double mutant. To examine this hypothesis, we crossed the zpr3-2 zpr4-2 mutant with a phb mutant. Although we screened >900 F2 progeny by genomic PCR, we were unable to obtain the zpr3-2 zpr4-2 phb triple mutant. This may be because the ZPR4 and PHB genes are located close to each other on the same chromosome (only 0.57 megabases apart). Because PHB and PHV functionally overlap, at least in part, we decided to generate the zpr3-2 zpr4-2 phv triple mutant. We found that the zpr3-2 zpr4-2 phenotype was moderately recovered in the triple mutant (Figure 6). The leaf number was reduced in the zpr3-2 zpr4-2 phv mutant, comparable to that in control plants. In addition, the triple mutant flowered earlier than the zpr3-2 zpr4-2 double mutant. These results indicate that the zpr3-2 zpr4-2 phenotype is caused at least in part by the enhanced PHV activity, further supporting the genetic relationship between ZPR3 and PHV. This genetic hierarchy would also be applicable to PHB.
Figure 6.
Phenotype of phv Compared with the Wild Type and zpr3-2 zpr4-2.
Note that the leaf number of the triple mutant is similar to that of the wild type control plant. Five-week-old plants were photographed.
The zpr4-1 mutant (see Supplemental Figure 12 online) also was crossed with the zpr3-2 mutant. The zpr3-2 zpr4-1 mutant showed a phenotype similar to that of the zpr3-2 zpr4-2 double mutant (data not shown).
zpr3-2 zpr4-2 Exhibits Homeotic Transformation and Ectopic Meristem Activity
The zpr3-2 zpr4-2 double mutant produced more flowers than the wild type and had clustered siliques like those observed in the phb-1d inflorescence (Figure 4H). These phenotypes would be related to the phenotypic similarity between the 35S:ZPR3 transgenic plants and the loss-of-function HD-ZIP III mutants, such as phb phv rev (Emery et al., 2003) in that both exhibit no functional meristem and stunted cotyledon development. Transgenic plants overexpressing ZPR4 also showed similar phenotypes to those of phb phv rev (see Supplemental Figure 13 online).
Interestingly, some zpr3-2 zpr4-2 flowers exhibited disrupted floral architectures with multiple carpels and tiny stamens (Figure 5D). Ovule-like structures were also formed on the outer surface of the ovaries in some flowers (Figure 5E), and papillae-like structures were observed at the petal margin (Figure 5F). Such homeotic transformations could be caused either by altered SAM activity (Okamuro et al., 1996) or by defects in organ polarity (Eshed et al., 1999), although the underlying molecular mechanisms are currently unclear. Notably, the inflorescence stem of the mutant was fasciated (Figure 5G, top panel), and ectopic leaves were produced on the inflorescence stem surface (Figure 5G, bottom panel), suggesting that ectopic meristematic cells might be present. Together, these observations strongly support that ZPR3 and ZPR4 are closely linked to proper maintenance of meristemic cells.
Overexpression of ZPR3 led to partial abaxialization of leaves (Figure 1B). Furthermore, the transcription of several YABBY family members regulating lateral organ polarity (Siegfried et al., 1999), particularly INO (Villanueva et al., 1999), was also altered to some degree in the zpr3-2 zpr4-2 double mutant (see Supplemental Figure 14 online). Therefore, a question was whether ZPR3 is essential for leaf polarity. We examined the surfaces of the zpr3-2 zpr4-2 leaves by scanning electron microscopy. No differences were observed in the adaxial and abaxial surfaces compared with those of the control leaves (see Supplemental Figure 14 online), showing that ZPR3 is not essential for leaf polar patterning. However, it is still possible that ZPR3 plays a redundant role with other ZPR proteins, such as ZPR1 and ZPR2, in determining leaf polarity.
CLV3 Is Highly Expressed in zpr3-2 zpr4-2
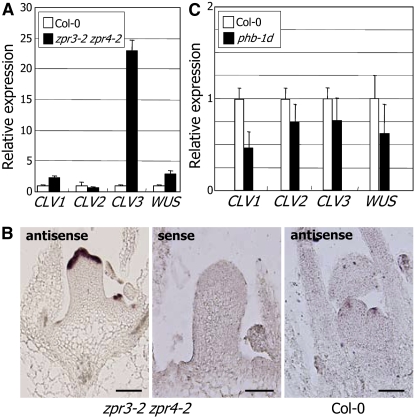
The WUSCHEL (WUS)–CLAVATA (CLV) feedback loop is an essential component of the molecular mechanisms governing SAM development (Williams and Fletcher, 2005). Since SAM activity was altered in the zpr3-2 zpr4-2 mutant, we examined the expression patterns of genes mediating SAM development. Transcription of CLV1 and WUS was elevated by 2 to 3 times in the mutant (Figure 7A). The CLV3 transcript level increased by more than 20 times, suggesting that CLV3 activation may contribute to the zpr3-2 zpr4-2 phenotype. This notion is also consistent with the previous observation that transgenic plants overexpressing CLV3 show a SAM phenotype similar to that of the zpr3-2 zpr4-2 mutant (Brand et al., 2000).
Figure 7.
Altered Expression Patterns of SAM-Related Genes in zpr3-2 zpr4-2.
(A) Expression of CLVs and WUS in zpr3-2 zpr4-2 compared with the wild type. Transcript levels were measured by real-time RT-PCR. Shoot apex tissues of 2-week-old plants were used for total RNA extractions. Means + se are shown (n = 3).
(B) In situ hybridization showing the expression domains of CLV3 in the zpr3-2 zpr4-2 apex compared with the wild type. The apex region was sectioned and probed either with a sense (negative control) or an antisense CLV3 probe. Bars = 50 μm.
(C) Expression of CLVs and WUS in phb-1d compared with the wild type. Transcript levels were measured by real-time RT-PCR. Shoot apex tissues of 2-week-old plants were used for total RNA extractions. Means + se are shown (n = 3).
The expression domain of CLV3 was also expanded to the entire apical dome of the SAM-like structure in the zpr3-2 zpr4-2 mutant, as verified by in situ hybridization assays (Figure 7B). In some mutants, it was further expanded to the side of the SAM-like structure and to the whole apex area (Figure 7B), suggesting that ZPR activities are related to both the expression levels and the expression domains of CLV3.
To examine whether the elevated expression of CLV3 in the zpr3-2 zpr4-2 mutant is due to enhanced activities of the HD-ZIP III proteins, we measured the transcript levels of CLV3 and other SAM-related genes in phb-1d. CLV1 was repressed by approximately twofold, and other CLV genes and WUS were also slightly repressed (Figure 7C). The upregulation of CLV3 in the zpr3-2 zpr4-2 mutant might result from SAM malfunctioning or reflect crosstalk of multiple signals mediating the HD-ZIP III activities in SAM development.
ZPR3 Regulates PHB via Negative Feedback
We observed that the transcript levels of the HD-ZIP III genes were unchanged in zpr3-1d (Figure 1D). It has been reported that the ZPR3 transcript levels are elevated in phb-1d and phv-1d (Wenkel et al., 2007), indicating that PHB positively regulates ZPR3 expression. Together with the negative regulation of PHB by ZPR3 at the protein level, these observations are consistent with ZPR3 and PHB being mutually regulated via negative feedback, as has been proposed (Wenkel et al., 2007).
Meanwhile, in situ hybridization assays (see Supplemental Figure 15 online) revealed that ZPR3 expression is confined to the CZ with a higher density in the organizing center, where WUS is also highly expressed. This observation shows that the expression domains of ZPR3 and PHB overlap (McConnell et al., 2001), further supporting the ZPR3 regulation of PHB.
DISCUSSION
Regulation of HD-ZIP III Activities by Competitive Inhibitors
Dynamic protein dimerization is a critical regulatory element in transducing diverse cellular signals (Klemm et al., 1998). The dominant-negative dimerization of transcription factors represents an example, as has been illustrated with the ID proteins in animals (Benezra et al., 1990; Ruzionova and Benezra, 2003; Perk et al., 2005).
Along with previous work (Wenkel et al., 2007), our data strongly support that there are extensive interactions between ZPR and HD-ZIP III proteins. Interestingly, the transcription of the ZPR genes is upregulated by the activation of the HD-ZIP III genes (Wenkel et al., 2007; this work), forming a negative feedback loop (Figure 8).
Figure 8.
A Schematic Model for ZPR3 Function.
ZPR3 negatively regulates HD-ZIP IIIs at the protein level. However, it is positively regulated by the HD-ZIP III genes at the transcriptional level, forming a negative feedback loop. miR165/166 does not directly interact with ZPR3 but regulates SAM development via the HD-ZIP IIIs.
HD-ZIP III transcription factors play diverse roles in lateral organ polarity, vascular development, and SAM development. The HD-ZIP III genes exhibit shared and distinct tissue-specific expression patterns during embryogenesis and lateral organ development. For example, PHB and PHV initiate their expression in the early stage of embryogenesis, but the expression domains are confined to the adaxial domain of the cotyledons and the central SAM (McConnell et al., 2001; Emery et al., 2003). REV is similarly expressed in the adaxial domain of lateral organs and vasculature (Otsuga et al., 2001; Emery et al., 2003).
An important molecular partner for HD-ZIP III function is miR165/166, which negatively regulates HD-ZIP III family members by cleaving their mRNAs (Juarez et al., 2004; Kidner and Martienssen, 2004; Kim et al., 2005; Williams et al., 2005). Since both the ZPR proteins and the miR165/166 negatively regulate the HD-ZIP III activities, it was envisioned that the ZPR proteins might be functionally related to miR165/166. As expected, the zpr3-1d mutant was phenotypically similar to the miR166-overproducing plants (Jung and Park, 2007). A similar result has also been reported with the transgenic plants overexpressing ZPR1 and ZPR3 (Wenkel et al., 2007). Accordingly, a triple mutant phb phv rev had an arrested SAM and pin-like leaves due to disrupted SAM activity (Emery et al., 2003).
Although the miR165/166 and the ZPR proteins have targets in common, it seems that they play both shared and discrete roles in SAM formation and determination of lateral polarity. Mutants overproducing miR166 exhibit radialized leaves and disrupted vascular tissues in addition to SAM enlargement (Kim et al., 2005; Williams et al., 2005). The most prominent phenotype of phb-1d and phv-1d is the severe malformation of lateral organs (leaf adaxialization and radial symmetry in severe cases), although they also exhibit an enlarged SAM. The phb phv rev triple mutants lack meristems and produce pin-shaped cotyledons. In addition, no leaves are produced. We observed that the zpr3-2 zpr4-2 leaves exhibit essentially normal leaf polarity but severely disturbed SAM activity. In addition, zpr3-1d lacks a visible SAM and has partially abaxialized leaves. We therefore propose that the regulatory role of miR165/166 is more prominent in the lateral organ patterning, whereas ZPR activity is more substantial in the SAM formation.
The structural organization of the HD-ZIP III proteins sheds lights on the molecular mechanisms by which ZPR activities are regulated. The central StAR-related lipid transfer (START) domain serves as a versatile binding interface for sterols and lipid molecules in animals (Ponting and Aravind, 1999). Although no ligands have been identified for the plant START domain yet, it is possible that certain hydrophobic molecules, such as brassinosteroids and unidentified lipids participating in signaling, may bind to the START domain and modulate the formation of heterodimers and/or homodimers. Another structural motif unique to the HD-ZIP III proteins is the MEKHLA motif located in their far C-terminal regions (Mukherjee and Bürglin, 2006). This motif is present exclusively in the class III members. Interestingly, the MEKHLA motif is structurally similar to the PAS (Per/Arnt/Sim) motif that mediates diverse protein–protein interactions (Crosson and Moffat, 2002). External signals and intrinsic developmental cues may affect the conformation of the HD-ZIP III proteins, allowing ZPR3 to recognize them and form nonfunctional heterodimers.
HD-ZIP III Regulators in SAM Function
Our data demonstrated that ZPR3 and ZPR4 play a key role in regulating SAM development. While the SAM was apparently disrupted in the zpr3-2 zpr4-2 mutant, ectopic meristem activities were observed in several locations, suggesting that the two ZPR members are involved both in proper maintenance of SAM activity and in suppressing meristem activity in nonstem cells. First, zpr3-2 zpr4-2 leaves were produced from the adaxial sides of leaf bases and the flat regions around the SAM-like structure (Figures 5A and 5B), rather than from the central SAM. Second, some flowers were also produced from the protrusions and rumples around the SAM-like structure. Third, ectopic leaves were formed on the inflorescence stem surface in the zpr3-2 zpr4-2 mutant (Figure 5G). Interestingly, such ectopic meristem activities are also observed in the gain-of-function mutants of the HD-ZIP III genes. For example, avb1, which contains mutations in the miRNA target site of the REV gene, develops ectopic shoots from the inflorescence (Zhong and Ye, 2004). In addition, phb-1d also develops ectopic shoots on the undersides of the leaves (McConnell and Barton, 1998).
CLV3 expression is normally confined to a small region within the SAM. In the zpr3-2 zpr4-2 mutant, it was expanded to the entire apical dome of the SAM-like structure (Figure 7B), and its expression level was also greatly elevated (Figure 7A). However, it was not upregulated in phb-1d. One possible explanation is that ZPR3 and ZPR4 target all the HD-ZIP III members and therefore their activities are enhanced in the zpr3-2 zpr4-2 mutant, resulting in higher expression levels of CLV3. The activation of a single target, such as PHB, may not be sufficient for CLV3 induction. In addition, the ectopic SAM development in phb-1d is not as severe as that in the zpr3-2 zpr4-2 mutant. This is also consistent with the functional redundancy among the HD-ZIP III proteins in SAM development. The CLV3 transcript is readily detected in the meristems of the clv1-4 mutant. However, it is greatly reduced in the clv1-4 cna-1 mutant (Green et al., 2005). In addition, the cna-2 phb-13 phv-11 and cna-1 phb mutants exhibit CLV3-deficient phenotypes, suggesting that the HD-ZIP III genes may be positive regulators of CLV3 expression (Green et al., 2005; Prigge et al., 2005).
Another possibility is that PHB upregulates ZPR3 expression, which sequentially represses PHB in the phb-1d mutant through negative feedback. It is also possible that at least some of the phb-1d phenotypes might come from altered expression domains of PHB. While PHB mRNA is detected only in the adaxial region of wild-type leaves, it is also found in the abaxial domain of phb-1d mutant leaves (McConnell et al., 2001). Although PHB activity is elevated in both phb-1d and zpr3-2 zpr4-2, the locations of increased PHB activity could be different.
At least some of the zpr3-2 zpr4-2 phenotypes can be explained by the CLV3 induction. Transgenic plants overexpressing CLV3 are phenotypically similar to the zpr3-2 zpr4-2 mutant in that leaf production is ceased after the emergence of a few leaves but reinitiated later at random positions (Brand et al., 2000). However, other phenotypes, such as the disorganized SAM morphology, the ectopic leaf formation on stems, and the carpelloid floral organs, are not observed in the CLV3-overexpressing plants, suggesting that CLV3 is not the sole mediator of ZPR function.
Functional Diversity of the ZPR Proteins
The zpr3-1d mutant exhibited partially abaxialized leaves and disrupted SAM activity. Wenkel et al. (2007) have also shown that ZPR3 is expressed in the adaxial region of leaves and that transgenic plants overexpressing ZPR3 have abaxialized leaves, sometimes with trumpet-shaped morphology and downward curling, in addition to meristem termination in extreme cases. Transgenic plants overexpressing ZPR1 also showed abaxialized leaves but not trumpet-shaped leaves (Wenkel et al., 2007), suggesting that ZPR1 may play a major role in generating patterns of leaf polarity. We observed that the zpr3-2 zpr4-2 double mutant had normal leaf polarity, indicating that ZPR3 and ZPR4 are not essential for the determination of leaf polarity.
The ZPR proteins are structurally similar in that they possess a ZIP motif. However, phylogenetic analysis revealed that they can be classified into two distinct groups: one including ZPR1 and ZPR2 and the other including ZPR3 and ZPR4 (Wenkel et al., 2007; see Supplemental Figure 5 online). The two groups are also distinct in their molecular sizes and the location of the ZIP motifs within the proteins. ZPR1 and ZPR2 (93 and 105 residues, respectively) are larger than ZPR3 and ZRP4 (67 and 72 residues, respectively) mainly because ZPR1 and ZPR2 have N-terminal extensions. The N-terminal extensions may play a role in DNA binding (Wenkel et al., 2007). While the ZIP motif is located in the C-terminal regions of ZPR1 and ZPR2, it is located in the N-terminal regions of ZPR3 and ZPR4. This structural distinction, together with the phenotypes of the mutant and transgenic plants with altered ZPR expressions (Wenkel et al., 2007; this work), support the idea that, whereas ZPR3 and ZPR4 play a role principally in the SAM development, ZPR1 and ZPR2 are more closely linked to the patterning of leaf polarity. Extensive examination of various mutants, including the zpr1 zpr2 and zpr3 zpr4 double mutants, and transgenic plants overexpressing the individual ZPR genes would clarify this hypothesis.
METHODS
Plant Materials and Growth Conditions
All Arabidopsis thaliana lines used were in the Col-0 ecotype, unless specified otherwise. Plants were grown in a culture room controlled at 22°C with relative humidity of 50% under long-day conditions (16 h light/8 h dark). White light illumination (110 μmol photons/m2s) was provided by FLR40D/A fluorescent tubes (Osram).
The 35S:ZPR3 transgenic plants were generated by expressing the ZPR3 gene under the control of the CaMV promoter in the pBA002 vector (Kost et al., 1998). An estradiol-inducible promoter was also used to direct ZPR3 expression, as previously described (Zuo et al., 2000). The 35S:REV transgenic plants were generated using the pK7WG2D Gateway vector (Karimi et al., 2002).
We generated transgenic plants overexpressing a MYC-PHB fusion, in which six copies of the MYC epitope tag-coding sequence were fused in frame to the PHB gene, under the CaMV 35S promoter using the pBA002 vector (Kim et al., 2006).
Isolation of Mutants
Arabidopsis ecotype Col-0 was transformed with an activation tagging vector pSKI015 as previously described (Weigel et al., 2000). Collected F0 seeds were sown in soil, and a Finale solution (AgrEvo), which contains 5.78% Basta, was diluted 1000 times in water and sprayed twice a week. Among the herbicide-resistant transformants, a morphogenic mutant (zpr3-1d) showing retarded growth with altered leaf morphology was chosen for analysis.
The single insertion event of T-DNA in zpr3-1d was verified by genomic DNA gel blot hybridization using the 35S enhancer sequence as probe, followed by analysis of segregation ratios. The sequences flanking the T-DNA insertion site were determined by three-step thermal asymmetric interlaced PCR using the LB2 and AD2 primers (Liu et al., 1995; see Supplemental Table 1 online).
The knockout mutant phv (SAIL_899_C08) was isolated from a pool of T-DNA insertion lines (Syngenta Arabidopsis Insertion Library), and the phb mutant (SALK-021684) was isolated from a pool of T-DNA insertion lines deposited in the ABRC.
The knockout mutants zpr3-2 (SALK-076134) and zpr4-1 (SALK-008069) were isolated from a mutant pool of T-DNA insertion lines (ABRC). The zpr4-2 (SM_3_15428) mutant was isolated from the John Innes Center suppressor mutator lines deposited in the Nottingham Arabidopsis Stock Center.
The zpr3-2 zpr4-2 double mutant was generated by crossing the homozygous zpr4-2 mutant with the homozygous zpr3-2 mutant. To find the homozygous double mutants, the seeds from the cross were grown on MS-agar plates. Among the ∼570 seedlings examined, 40 plants had abnormal numbers of cotyledons. All the seedlings with more than two cotyledons were identified as double mutants. By contrast, all the 23 seedlings with two cotyledons examined were the parental mutants.
To obtain the zpr3-2 zpr4-2 phb phv quadruple mutant, we first crossed zpr3-2 with phb and zpr4-2 with phv. We subsequently crossed the zpr3-2/+ phb/+ plants with the zpr4-2/+ phv/+ plants. We examined the resulting F2 plants to isolate the quadruple (zpr3-2 zpr4-2 phb phv) and the triple (zpr3-2 zpr4-2 phv or zpr3-2 zpr4-2 phb) mutants. We screened >900 plants by genomic PCR. Genomic DNA was isolated using a genomic DNA isolation kit (iNtRON). The PCR primers for ZPR3 and ZPR4 and those for PHB and PHV (PHBg and PHVg, respectively) are described in Supplemental Table 1 online. We were able to obtain the zpr3-2 zpr4-2 phv triple mutant, but we could not identify the quadruple mutants and the zpr3-2 zpr4-2 phb triple mutant.
The zpr3-1d mutant was genetically crossed with the 35S:REV transgenic plants, and the zpr3-1d 35S:REV plants were obtained by selections with Basta and kanamycin.
The absence of gene expression was verified by RT-PCR in all the mutants obtained before use.
Analysis of Transcript Levels
Extraction of total RNA samples from appropriate plant materials and RT-PCR conditions have been previously described (Kim et al., 2006). Whenever possible, positive and negative control genes were included in the RT-PCR reactions to validate the assay conditions. The PCR primers for the HD-ZIP III genes have been previously described (Kim et al., 2005). Those newly synthesized in this work are listed in Supplemental Table 1 online.
For RT-PCR–based DNA gel blot hybridization, RT-PCR products were electrophoresed on 1 to 1.2% agarose gel and transferred to a Hybond-N+ nylon membrane (Amersham-Pharmacia). The membrane was hybridized at 65°C with gene-specific probes labeled with 32P-dCTP using the Megaprime DNA labeling system (Amersham-Pharmacia).
Quantitative real-time RT-PCR was performed in 96-well blocks with an Applied Biosystems 7500 real-time PCR system using the SYBR Green I master mix in a volume of 25 μL. The PCR primers (see Supplemental Table 1 online) were designed using the Primer Express Software installed into the system. The two-step thermal cycling profile used was 15 s at 94°C and 1 min at 68°C. A tubulin gene was included as an internal control for normalization of the variations in cDNA amounts used. Biological duplicates were performed, and the reactions were performed in triplicate for each run. The comparative ΔΔCT method was used to evaluate the relative quantities of each amplified product in the samples. The threshold cycle (CT) was automatically determined for each reaction by the system set with default parameters. The specificity of the PCR was determined by melt curve analysis of the amplified products using the standard method installed in the system.
Scanning Electron Microscopy
Appropriate plant materials were fixed in glutaraldehyde solution (3% glutaraldehyde in 25 mM phosphate buffer, pH 7.0) at 4°C for 24 h. The samples were subsequently incubated in 1% osmium tetroxide in 25 mM phosphate buffer, pH 7.0, at 4°C for several days. They were then subjected to critical point drying and scanning electron microscopy using model JSM 5410LV (JEOL) located in the National Instrumentation Center for Environmental Management (Seoul National University). To examine the leaf surfaces, the medial regions in the nonmarginal part of the leaves were photographed.
Yeast Two-Hybrid Assay
Yeast two-hybrid assays were performed using the BD Matchmaker system (Clontech). pGADT7 was used for GAL4 AD, and pGBKT7 was used for GAL4 BD. The yeast strain AH109 (leu-, trp-, ade-, his-), with chromosomally integrated reporter genes lacZ and HIS under the control of the GAL1 promoter that is activated by the GAL4 transcription factor, was used for transformation. ZPR3 and HD-ZIP III cDNAs were amplified by RT-PCR as previously described (Kim et al., 2005) using the primer sets described in Supplemental Table 1 online. The ZPR3 PCR product was digested with EcoRI and SalI and subcloned into pGBKT7. The HD-ZIP III PCR products were digested with restriction enzymes (PHB with NdeI and SacI, PHV with EcoRI and BamHI, REV with NdeI and SacI, ATHB8 with XhoI and SmaI, and ATHB15 with EcoRI and BamHI) and subcloned into pGADT7. Transformation of the AH109 cells (Clontech) was performed according to the manufacturer's instructions. Colonies obtained were streaked on a medium without His, Ade, Leu, and Trp. To confirm the results, β-Gal assays were performed according to the system procedure.
The pBridge vector (Clontech) was used for yeast three-hybrid screening. A truncated PHB gene sequence missing the C-terminal coding sequence (PHB-ΔC) was amplified by RT-PCR, and the PCR products were digested with EcoRI and SalI and subcloned into the pBridge vector to generate the BD-ΔC construct. The ZPR3 gene was subcloned into the NotI/BglII-digested pBridge vector so that its expression was controlled by the pMET25 promoter. The expression constructs (BD-ΔC and MET25:ZPR3 in the pBridge vector and AD-PHB in the pGADT7 vector) were cotransformed into the AH109 cells. The colonies were streaked on media without Leu, Trp, and His supplemented with or without Met.
Subcellular Localization Assays
The GFP-coding sequence was fused in frame to the 3′ end of the ZPR3 gene by subcloning the ZPR3 gene into the pCAMBIA1304 vector (CAMBIA). The ZPR3-GFP fusion sequence was amplified by PCR using the primer pair ZPR3-GFP (see Supplemental Table 1 online), and the PCR product was digested with XbaI and PacI and subcloned into the pBA002 vector (Kost et al., 1998) for transient expression in onion epidermal cells. Transient expression was achieved by particle bombardment as previously described (Klein et al., 1987).
After incubation for 24 h at 23°C, the cells were subject to bright-field and fluorescence microscopy.
In Vitro Pull-Down Assays
Recombinant GST and GST-ZPR3 proteins were expressed in Escherichia coli BL21-CodonPlus (DE3)-RIL strains (Stratagene) and purified as follows. One-tenth volume of precultured cells (5 mL) was added to 500 mL of fresh Luria-Bertani medium and cultured at 37°C until the OD600 reached 0.3 to 0.6. Protein production was induced by adding isopropyl-β-d-thiogalactopyranoside at a final concentration of 0.5 mM and shaking at 37°C for 5 h. Cells were harvested and resuspended in buffer A (25 mM HEPES, pH 7.5, 20% glycerol, 1 mM DTT, 100 mM NaCl, 0.2 mM EDTA with protease inhibitor cocktail [Sigma-Aldrich], and 1 mM PMSF). The cells were lysed by a French press (8500 p.s.i.; one time). The lysates were sonicated for 30 s twice and centrifuged at 20,000g for 20 min. The supernatants were stored at −80°C until use. The HD-ZIP III and ATHB2 genes were amplified by PCR. For subcloning into the pGADT7 vector, the PCR products were linearized with appropriate restriction enzymes (NdeI/SacI for PHB and REV, EcoRI/BamHI for PHV and ATHB15, SmaI/PstI for ATHB8, and EcoRI/ClaI for ATHB2) and purified by phenol extraction and ethanol precipitation. The HD-ZIP III and ATHB2 polypeptides were labeled with 35S-Met using the TNT coupled reticulocyte lysate system (Promega).
The GST or GST-ZPR3 proteins were mixed with glutathione agarose beads (Sigma-Aldrich) and agitated for 15 min at room temperature. The beads were then washed three times with 1× PBS and one time with buffer A. Five microliters of the 35S-labeled proteins was added and incubated for 2 h at 4°C. The beads were then washed five times with buffer A. The bound proteins were eluted with 1× SDS loading buffer by boiling for 5 min at 100°C and subjected to SDS-PAGE and autoradiography.
ZPR3-PHB Interaction Assays in Nicotiana benthamiana Cells
The miR165/166-resistant mPHB and mREV mutant genes have been previously described (Kim et al., 2005). Direct infiltration of N. benthamiana leaves was performed as previously described (Kim et al., 2006). Total proteins containing MYC-mPHB, MYC-mPHV, or MYC-ATHB2 were extracted with extraction buffer (50 mM HEPES, pH 7.9, 20% glycerol, 1 mM DTT, 100 mM NaCl, and 0.2 mM EDTA) containing a protease inhibitor cocktail (Sigma-Aldrich). The protein extracts were incubated with GST-conjugated agarose beads at 4°C for 3 h. The beads were then washed four times with fresh extraction buffer supplemented with 0.1% Triton X-100, and the bound proteins were eluted with 1× SDS-PAGE loading buffer. The eluted proteins (mPHB, mPHV, or ATHB2) were subjected to SDS-PAGE and transferred to a polyvinylidene fluoride membrane (Amersham-Pharmacia). The HD-ZIP III proteins were detected by protein gel blot analysis using an anti-MYC antibody (Upstate).
Histological Analysis
Seedlings were embedded in paraplast (Oxford Labware). Eight-micrometer sections were prepared using the Leica RM2125RT microtome, stained in 0.05% toluidine blue, and examined with a Nikon Microphot FXA microscope.
RNA in Situ Hybridizations
RNA in situ hybridization was performed as previously described (Jackson, 1991) with minor modifications. Ten-day-old Arabidopsis seedlings were fixed in 4% formaldehyde. The embedded tissues were sectioned to a thickness of 8 μm. The ZPR3 cDNA was amplified by RT-PCR (30 cycles, each polymerization at 55°C for 30 s) using a primer pair, ZPR3-in situ (see Supplemental Table 1 online), and the EcoRI-digested PCR fragment was subcloned into the pGEM-7Zf(+) vector (Promega). The orientation of the insert was determined by DNA sequencing. To generate an antisense probe, the plasmid was digested with XbaI, and DIG-UTP–labeled RNA was generated using SP6 RNA polymerase. A sense probe was similarly prepared using ClaI and T7 RNA polymerase. All of the hybridizations and washes were performed at 52°C.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: ZPR1 (At2g45450), ZPR2 (At3g60890), ZPR3 (At3g52770), ZPR4 (At2g36307), ATHB15 (At1g52150), ATHB8 (At4g32880), REV (At5g60690), PHB (At2g34710), PHV (At1g30490), ATHB2 (At4g16789), and CLV3 (At2g27250).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Comparison of Leaf Morphologies and Structures.
Supplemental Figure 2. Reduced Secondary Shoots in zpr3-1d.
Supplemental Figure 3. Trumpet-Shaped Cotyledons and Pin-Like Structure at the zpr3-1d Apex.
Supplemental Figure 4. ZPR3 (At3g52770) Activation in zpr3-1d.
Supplemental Figure 5. Multiple Sequence Alignment of ZPR Proteins.
Supplemental Figure 6. Phenotypes of Transgenic Plants Overexpressing ZPR3 (35S:ZPR3).
Supplemental Figure 7. Interactions of ZPR with HD-ZIP IIIs in Yeast Cells.
Supplemental Figure 8. In vitro Pull-Down Assays.
Supplemental Figure 9. Phenotypic Comparison of phb-1d and phb-1d-Col.
Supplemental Figure 10. RT-PCR Analysis of ZPR3 and REV Expression in the 35S:REV and 35S:REV zpr3-1d Plants.
Supplemental Figure 11. Complementation of the zpr3-2 and zpr3-2 zpr4-2 Mutants.
Supplemental Figure 12. Isolation of the zpr4 Knockout Mutants.
Supplemental Figure 13. Phenotypic Comparison of the 35S:ZPR4 Transgenic and the phb phv rev Mutant Seedlings.
Supplemental Figure 14. Altered Expression Patterns of Leaf Polarity Patterning Genes and Scanning Electron Microscopy Analysis of the Leaf Surfaces in the zpr3-2 zpr4-2 Mutant.
Supplemental Figure 15. Expression Domains of ZPR3 in the Apex of Control Plants.
Supplemental Table 1. Primers Used in This Work.
Supplementary Material
Acknowledgments
We thank D. Weigel for kindly providing the pSKI015 vector. This work was supported by Brain Korea 21, Biogreen 21 (20080401034001) and National Research Laboratory Programs and by grants from the Plant Signaling Network Research Center, Korea Research Foundation (2005-070-C00129) and from the Korea Science and Engineering Foundation (2007-03415).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Chung-Mo Park (cmpark@snu.ac.kr).
Online version contains Web-only data.
References
- Baima, S., Nobili, F., Sessa, G., Lucchetti, S., Ruberti, I., and Morelli, G. (1995). The expression of the ATHB-8 homeobox gene is restricted to provascular cells in Arabidopsis thaliana. Development 121 4171–4182. [DOI] [PubMed] [Google Scholar]
- Benezra, R., Davis, R.L., Lockshon, D., Turner, D.L., and Weintraub, H. (1990). The protein ID: A negative regulator of helix-loop-helix DNA binding proteins. Cell 61 49–59. [DOI] [PubMed] [Google Scholar]
- Bowman, J.L. (2004). Class III HD-ZIP gene regulation, the golden fleece of ARGONAUTE activity? Bioessays 26 938–942. [DOI] [PubMed] [Google Scholar]
- Brand, U., Fletcher, J.C., Hobe, M., Meyerowitz, E.M., and Simon, R. (2000). Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science 289 617–619. [DOI] [PubMed] [Google Scholar]
- Byrne, M.E. (2006). Shoot meristem function and leaf polarity: The role of class III HD-ZIP genes. PLoS Genet. 2 785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraro, N., Peaucelle, A., Laufs, P., and Traas, J. (2006). Cell differentiation and organ initiation at the shoot apical meristem. Plant Mol. Biol. 60 811–826. [DOI] [PubMed] [Google Scholar]
- Crosson, S., and Moffat, K. (2002). Photoexcited structure of a plant photoreceptor domain reveals a light-driven molecular switch. Plant Cell 14 1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery, J.F., Floyd, S.K., Alvarez, J., Eshed, Y., Hawker, N.P., Izhaki, A., Baum, S.F., and Bowman, J.L. (2003). Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr. Biol. 13 1768–1774. [DOI] [PubMed] [Google Scholar]
- Eshed, Y., Baum, S.F., and Bowman, J.L. (1999). Distinct mechanisms promote polarity establishment in carpels of Arabidopsis. Cell 99 199–209. [DOI] [PubMed] [Google Scholar]
- Eshed, Y., Baum, S.F., Perea, J.V., and Bowman, J.L. (2001). Establishment of polarity in lateral organs of plants. Curr. Biol. 11 1251–1260. [DOI] [PubMed] [Google Scholar]
- Golz, J.F. (2006). Signalling between the shoot apical meristem and developing lateral organs. Plant Mol. Biol. 60 889–903. [DOI] [PubMed] [Google Scholar]
- Green, K.A., Prigge, M.J., Katzman, R.B., and Clark, S.E. (2005). CORONA, a member of the class III homeodomain-leucine zipper gene family in Arabidopsis, regulates stem cell specification and organogenesis. Plant Cell 17 691–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun, Y., and Lee, I. (2006). KIDARI, encoding a non-DNA binding bHLH protein, represses light signal transduction in Arabidopsis thaliana. Plant Mol. Biol. 61 283–296. [DOI] [PubMed] [Google Scholar]
- Jackson, D. (1991). In situ hybridization in plants. In Molecular Plant Pathology: A Practical Approach, D.J. Bowles, S.J. Gurr, and M. McPherson, eds (London: Oxford University Press), pp. 157–181.
- Juarez, M.T., Kui, J.S., Thomas, J., Heller, B.A., and Timmermans, M.C. (2004). MicroRNA-mediated repression of rolled leaf1 specifies maize leaf polarity. Nature 428 84–88. [DOI] [PubMed] [Google Scholar]
- Jung, J.-H., and Park, C.-M. (2007). MIR165/166 genes exhibit dynamic expression patterns in regulating shoot apical meristem and floral development in Arabidopsis. Planta 225 1327–1338. [DOI] [PubMed] [Google Scholar]
- Karimi, M., Inze, D., and Depicker, A. (2002). GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 5 193–195. [DOI] [PubMed] [Google Scholar]
- Kerstetter, R.A., Bollman, K., Tayler, R.A., Bomblies, K., and Poethig, R.S. (2001). KANADI regulates organ polarity in Arabidopsis. Nature 411 706–709. [DOI] [PubMed] [Google Scholar]
- Kidner, C.A., and Martienssen, R.A. (2004). Spatially restricted microRNA directs leaf polarity through ARGONAUTE1. Nature 428 81–84. [DOI] [PubMed] [Google Scholar]
- Kim, J., Jung, J.H., Reyes, J.L., Kim, Y.S., Kim, S.Y., Chung, K.S., Kim, J.A., Lee, M., Lee, Y., Narry Kim, V., Chua, N.H., Park, C.M. (2005). MicroRNA-directed cleavage of ATHB15 mRNA regulates vascular development in Arabidopsis inflorescence stems. Plant J. 42 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y.S., Kim, S.G., Park, J.E., Park, H.Y., Lim, M.H., Chua, N.-H., and Park, C.-M. (2006). A membrane-bound NAC transcription factor regulates cell division in Arabidopsis. Plant Cell 18 3132–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, T.M., Wolf, E.D., Wu, R., and Sanford, J.C. (1987). High velocity microprojectiles for delivering nucleic acids into living cells. Nature 327 70–73. [PubMed] [Google Scholar]
- Klemm, J.D., Schreiber, S.L., and Crabtree, G.R. (1998). Dimerization as a regulatory mechanism in signal transduction. Annu. Rev. Immunol. 16 569–592. [DOI] [PubMed] [Google Scholar]
- Kost, B., Spielhofer, P., and Chua, N.-H. (1998). A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes. Plant J. 16 393–401. [DOI] [PubMed] [Google Scholar]
- Li, H., Xu, L., Wang, H., Yuan, Z., Cao, X., Yang, Z., Zhang, D., Xu, Y., and Huang, H. (2005). The putative RNA-dependent RNA polymerase RDR6 acts synergistically with ASYMMETRIC LEAVES1 and 2 to repress BREVIPEDICELLUS and microRNA165/166 in Arabidopsis leaf development. Plant Cell 17 2157–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y.G., Mitsukawa, N., Oosumi, T., and Whittier, R.F. (1995). Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J. 8 457–463. [DOI] [PubMed] [Google Scholar]
- Llave, C., Xie, Z., Kasschau, K.D., and Carrington, J.C. (2002). Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science 297 2053–2056. [DOI] [PubMed] [Google Scholar]
- McConnell, J.R., and Barton, M.K. (1998). Leaf polarity and meristem formation in Arabidopsis. Development 125 2935–2942. [DOI] [PubMed] [Google Scholar]
- McConnell, J.R., Emery, J., Eshed, Y., Bao, N., Bowman, J.L., and Barton, M.K. (2001). Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature 411 709–713. [DOI] [PubMed] [Google Scholar]
- Mukherjee, K., and Bürglin, T.R. (2006). MEKHLA, a novel domain with similarity to PAS domains, is fused to plant homeodomain-leucine zipper III proteins. Plant Physiol. 140 1142–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira, F.T.S., Madi, S., Chitwood, D.H., Juarez, M.T., and Timmermans, M.C.P. (2007). Two small regulatory RNAs establish opposing fates of a developmental axis. Genes Dev. 21 750–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamuro, J.K., den Boer, B.G., Lotys-Prass, C., Szeto, W., and Jofuku, K.D. (1996). Flowers into shoots: photo and hormonal control of a meristem identity switch in Arabidopsis. Proc. Natl. Acad. Sci. USA 93 13831–13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen, K., Johannesen, P.K., Hoffmann, L., Sorensen, S.B., Gjermansen, C., and Hansen, J. (2000). The pYC plasmids, a series of cassette-based yeast plasmid vectors providing means of counter-selection. Yeast 16 1035–1043. [DOI] [PubMed] [Google Scholar]
- Otsuga, D., DeGuzman, B., Prigge, M.J., Drews, G.N., and Clark, S.E. (2001). REVOLUTA regulates meristem initiation at lateral positions. Plant J. 25 223–236. [DOI] [PubMed] [Google Scholar]
- Perk, J., Iavarone, A., and Benezra, R. (2005). ID family of helix-loop-helix proteins in cancer. Nat. Rev. Cancer 5 603–614. [DOI] [PubMed] [Google Scholar]
- Ponting, C.P., and Aravind, L. (1999). START: A lipid-binding domain in StAR, HD-ZIP and signaling proteins. Trends Biochem. Sci. 24 130–132. [DOI] [PubMed] [Google Scholar]
- Prigge, M.J., Otsuga, D., Alonso, J.M., Ecker, J.R., Drews, G.N., and Clark, S.E. (2005). Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell 17 61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt, D., Frenz, M., Mandel, T., and Kuhlemeier, C. (2005). Microsurgical and laser ablation analysis of leaf positioning and dorsoventral patterning in tomato. Development 132 15–26. [DOI] [PubMed] [Google Scholar]
- Ruberti, I., Sessa, G., Lucchetti, S., and Morelli, G. (1991). A novel class of plant proteins containing a homeodomain with a closely linked leucine zipper motif. EMBO J. 10 1787–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzionova, M.B., and Benezra, R. (2003). ID proteins in development, cell cycle and cancer. Trends Cell Biol. 13 410–418. [DOI] [PubMed] [Google Scholar]
- Sessa, G., Steindler, C., Morelli, G., and Ruberti, I. (1998). The Arabidopsis Athb-8, -9, and -14 are members of a small gene family coding for highly related HD-ZIP proteins. Plant Mol. Biol. 38 609–622. [DOI] [PubMed] [Google Scholar]
- Siegfried, K.R., Eshed, Y., Baum, S.F., Ostuga, D., Drews, G.N., and Bowman, J.L. (1999). Members of the YABBY gene family specify abaxial cell fate in Arabidopsis. Development 126 4117–4128. [DOI] [PubMed] [Google Scholar]
- Talbert, P.B., Adler, H.T., Parks, D.W., and Comai, L. (1995). The REVOLUTA gene is necessary for apical meristem development and for limiting cell divisions in the leaves and stems of Arabidopsis thaliana. Development 121 2723–2735. [DOI] [PubMed] [Google Scholar]
- Vaucheret, H., Vazquez, F., Crété, P., and Bartel, D.P. (2004). The action of ARGONAUTE1 in the miRNA pathway and its regulation by the miRNA pathway are crucial for plant development. Genes Dev. 18 1187–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva, J.M., Broadhvest, J., Hauser, B.A., Meister, R.J., Schneitz, K., and Gasser, C.S. (1999). INNER NO OUTER regulates abaxial- adaxial patterning in Arabidopsis ovules. Genes Dev. 13 3160–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel, D., et al. (2000). Activation tagging in Arabidopsis. Plant Physiol. 122 1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenkel, S., Emercy, J., Hou, B., Evans, M.M.S., and Barton, M.K. (2007). A feedback regulatory module formed by LITTLE ZIPPER and HD-ZIPIII genes. Plant Cell 19 3379–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, L., and Fletcher, J.C. (2005). Stem cell regulation in the Arabidopsis shoot apical meristem. Curr. Opin. Plant Biol. 8 582–586. [DOI] [PubMed] [Google Scholar]
- Williams, L., Grigg, S.P., Xie, M., Christensen, S., and Fletcher, J.C. (2005). Regulation of Arabidopsis shoot apical meristem and lateral organ formation by microRNA miR166g and its AtHD-ZIP target genes. Development 132 3657–3668. [DOI] [PubMed] [Google Scholar]
- Zhong, R., and Ye, Z.H. (2004). amphivasal vascular bundle 1, a gain-of-function mutation of the IFL1/REV gene, is associated with alterations in the polarity of leaves, stems and carpels. Plant Cell Physiol. 45 369–385. [DOI] [PubMed] [Google Scholar]
- Zuo, J., Niu, Q.-W., and Chua, N.-H. (2000). An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 24 265–273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.