Abstract
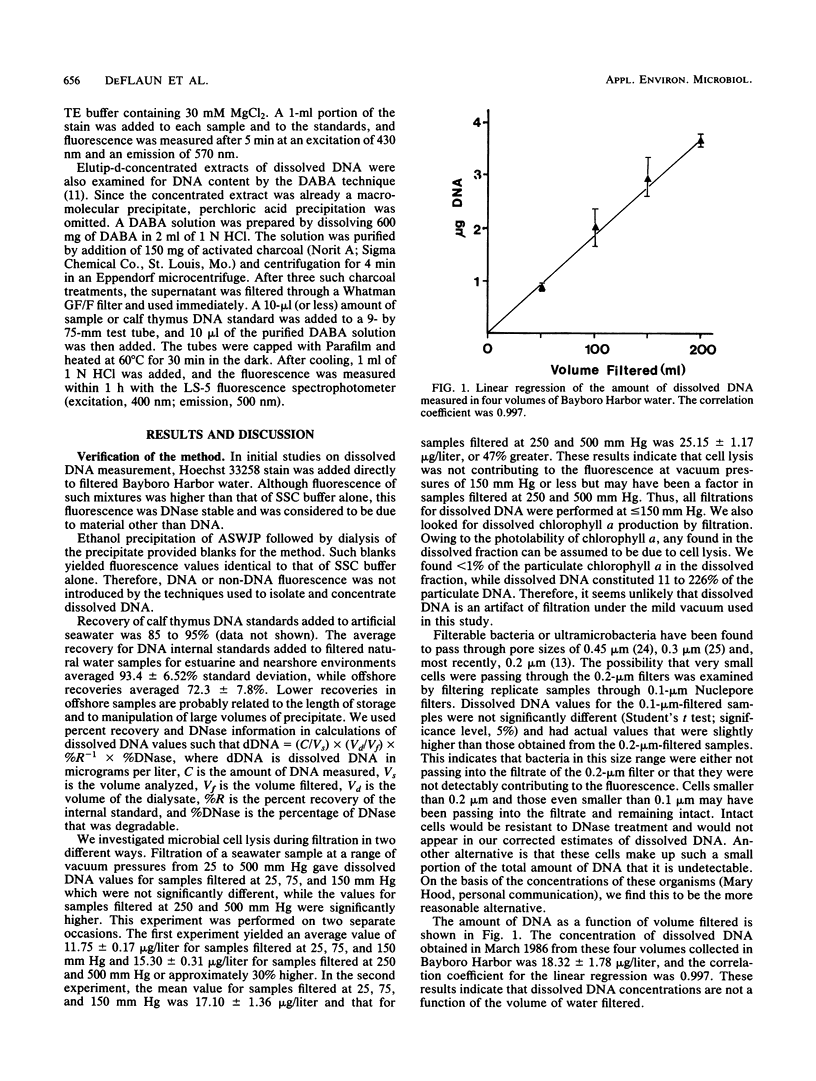
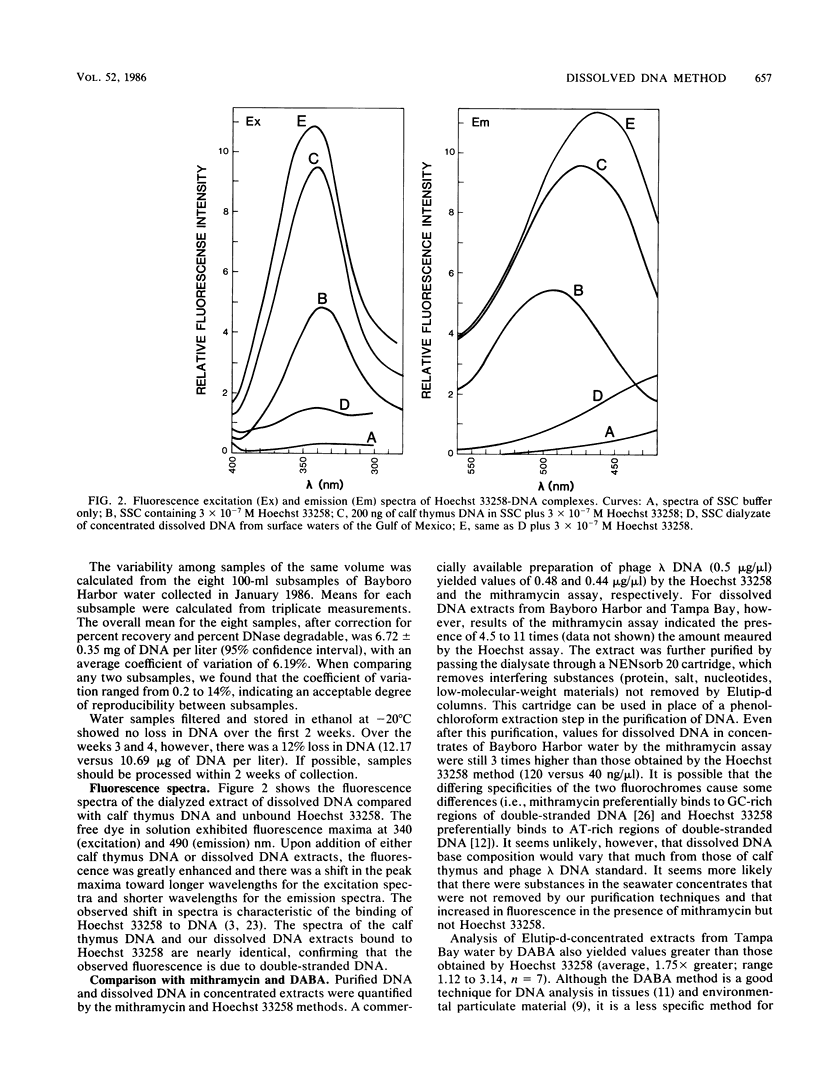
A method was developed for the determination of dissolved DNA in aquatic environments. The method is based upon the concentration of dissolved DNA by ethanol precipitation of 0.2-μm-pore-size filtered water. The DNA in concentrated extracts was quantified by the fluorescence of Hoechst 33258-DNA complexes. Fluorescence not attributable to DNA was corrected for by DNase I digestion of the extracts and averaged 25% of the total fluorescence for all samples. The effectiveness of the procedure for concentrating dissolved DNA was demonstrated by the efficient (>90%) recovery of internal standards. Concentrations of dissolved DNA from a variety of marine and freshwater environments ranged from 0.2 to 44 μg/liter, with the highest values being obtained for estuarine and river environments. The method is simple, specific for DNA, and more sensitive than previously described methods for the determination of extracellular DNA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cesarone C. F., Bolognesi C., Santi L. Improved microfluorometric DNA determination in biological material using 33258 Hoechst. Anal Biochem. 1979 Nov 15;100(1):188–197. doi: 10.1016/0003-2697(79)90131-3. [DOI] [PubMed] [Google Scholar]
- Hill B. T., Whatley S. A simple, rapid microassay for DNA. FEBS Lett. 1975 Aug 1;56(1):20–23. doi: 10.1016/0014-5793(75)80102-5. [DOI] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Latt S. A., Stetten G. Spectral studies on 33258 Hoechst and related bisbenzimidazole dyes useful for fluorescent detection of deoxyribonucleic acid synthesis. J Histochem Cytochem. 1976 Jan;24(1):24–33. doi: 10.1177/24.1.943439. [DOI] [PubMed] [Google Scholar]
- Macdonell M. T., Hood M. A. Isolation and characterization of ultramicrobacteria from a gulf coast estuary. Appl Environ Microbiol. 1982 Mar;43(3):566–571. doi: 10.1128/aem.43.3.566-571.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. E., Hodson R. E. Microbial biomass and utilization of dissolved organic matter in the okefenokee swamp ecosystem. Appl Environ Microbiol. 1984 Apr;47(4):685–692. doi: 10.1128/aem.47.4.685-692.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. H., Myers B. Fluorometric determination of DNA in aquatic microorganisms by use of hoechst 33258. Appl Environ Microbiol. 1982 Jun;43(6):1393–1399. doi: 10.1128/aem.43.6.1393-1399.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner R. F., Sternberg H. The interaction of Hoechst 33258 with natural and biosynthetic nucleic acids. Arch Biochem Biophys. 1979 Oct 15;197(2):580–588. doi: 10.1016/0003-9861(79)90282-0. [DOI] [PubMed] [Google Scholar]
- Torrella F., Morita R. Y. Microcultural study of bacterial size changes and microcolony and ultramicrocolony formation by heterotrophic bacteria in seawater. Appl Environ Microbiol. 1981 Feb;41(2):518–527. doi: 10.1128/aem.41.2.518-527.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. K., Sasaki A. W., Matthews M. A., Wagner R. C. Quantitative determination of deoxyribonucleic acid from cells collected on filters. Anal Biochem. 1980 Sep 1;107(1):17–20. doi: 10.1016/0003-2697(80)90485-6. [DOI] [PubMed] [Google Scholar]


