Abstract
Background and objectives: Children and adolescents with ESRD on dialysis are susceptible to serious bacterial infections (SBI). Chemokines and chemokine receptors play a critical role in modulating macrophage and neutrophil function. This study examined the hypothesis that expression and/or function of these molecules is dysregulated in patients with ESRD, contributing to leukocyte dysfunction.
Design setting, participants, & measurements: Pediatric patients, age 6 mo to 18 yr, with ESRD treated with either hemodialysis or peritoneal dialysis were enrolled in this prospective, nontherapeutic study. Blood was collected for plasma chemokine levels, chemokine receptor profiling by flow cytometry, and functional chemotaxis studies on neutrophils and mononuclear cells.
Results: ESRD in children was associated with reduced expression of the chemokine receptors CXCR1 and chemokine (C-C motif) receptor 2 (CCR2) on circulating neutrophils and monocytes, respectively. When ESRD patients were divided into two subgroups, those who were infection-free and those who had three or more SBI in the preceding year, the differences in chemokine receptor expression were statistically significant compared with control subjects only in those with recurrent infection. In addition to the effects of ESRD on baseline chemokine receptor expression, the hemodialysis procedure itself acutely lowered neutrophil CXCR1 and monocyte CCR2 expression. Furthermore, neutrophil and monocyte responsiveness to chemokine-mediated trafficking signals was impaired in all ESRD patients studied. This abnormality was independent of the level of chemokine receptor expression on the leukocytes.
Conclusions: The data presented in this study suggest that chemokine receptor dysregulation contributes to leukocyte dysfunction in patients with ESRD. This alteration is especially prominent in ESRD patients with recurrent infection.
Infectious complications cause significant morbidity in patients with ESRD on dialysis (1,2). The costs include hospitalization, administration of antibiotics, and surgical procedures to correct malfunction of the dialysis access device (1). Moreover, SBI are the second most common cause of death in this patient population (1). Most infections are related to the use of prosthetic appliances needed for the dialysis procedure.
In pediatric patients with ESRD who require hemodialysis, an increasing number are being dialyzed using tunneled, dual-lumen catheters that can be maintained within a central blood vessel for up to one year (3). The frequency of catheter-related infections has been estimated to be 3 to 5.5 per 1000 patient days, equivalent to 0.7 to 1.5 infections per catheter per year (1,3,4). The occurrence rate follows a bimodal distribution with one group having rare or no infections and a second group with fairly frequent episodes (1,4). Peritoneal dialysis is an alternative means of dialyzing pediatric patients with ESRD. However, a major limitation of peritoneal dialysis is peritonitis, and exit site or tunnel infections. The rate of infection is approximately one episode per 10-mo of patient use (5,6). Similar to the pattern of infection in children on hemodialysis, there are two distinct patient populations, one with recurrent episodes of peritonitis and one with virtually no infections.
Several investigations have assessed immune function in patients with ESRD on dialysis. The best-documented abnormalities in immune function are diminished bacterial phagocytosis by polymorphonuclear leukocytes (neutrophils) and impaired clearance of IgG-coated erythrocytes by monocytes (7).
Members of the chemokine family play a pivotal role in eliciting the migration of specific subsets of inflammatory cells, a hallmark of effective host defense (8,9). The chemokines interleukin-8 (IL-8), groα, groβ, and groγ modulate the trafficking and activation state of neutrophils via binding and signaling through their cognate receptors, CXCR1 and CXCR2. Monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), macrophage inflammatory protein-1β (MIP-1β), and regulated upon activation, normal T cell expressed, and secreted (RANTES) protein modulate the trafficking and activation status of macrophages via binding and signaling through CCR1, CCR2, and CCR5 (10). Impaired expression of chemokine and/or chemokine receptors (either via gene knockout or mutation) leads to increased susceptibility to infection (11–18). Therefore in this prospective, nontherapeutic study we examined whether there are abnormalities in plasma chemokine levels and/or cell-associated chemokine receptors in pediatric patients with ESRD on dialysis leading to diminished neutrophil and monocyte/macrophage function. In addition, we determined if these changes were associated with a heightened risk of developing SBI such as catheter-related sepsis.
Concise Methods
Reagents and Antibodies
Monoclonal anti-CCR5 (clone 2D7) labeled with phytoerythrin, anti-CD14 labeled with allophycocyanin (APC), appropriate isotype controls, APC beads, and red blood cell lysis buffer were obtained from BD PharMingen (San Diego, California). Monoclonal antibodies against CCR1, CCR2, CXCR1, and CXCR2, and appropriate isotype controls all labeled with phytoerythrin were obtained from R&D Systems (Minneapolis, Minnesota). Calcein-AM was obtained from Molecular Probes (Eugene, Oregon).
Study Population
Patients enrolled in this prospective, nontherapeutic study were children and adolescents 6 mo to 18 yr of age who had ESRD and were being treated with either hemodialysis using a tunneled double-lumen catheter, arteriovenous (AV) fistula, or AV graft, or peritoneal dialysis using a Tenckhoff catheter between December 1, 2002 and April 5, 2007. Patients had hematocrit more than 30 and a stable erythropoietin dose for at least 8 wk before study entry. SBI included all catheter-related infections and peritonitis. Hemodialysis catheter-associated infection was defined as an episode of fever with a positive blood culture obtained via the catheter at the onset of hemodialysis. Peritonitis was defined by the presence of fever, abdominal pain, and a peritoneal fluid cell count exceeding 250. Healthy children and adolescents with a normal GFR were used as controls. All procedures in this study (Protocol #: 03-08-082) were in accordance with the ethical standards of The North Shore–Long Island Jewish Health System Office of the Institutional Review Board, and informed consent was obtained from all study subjects.
Blood Collection and Processing
Five ml of EDTA anticoagulated blood was collected from each study subject. In patients on hemodialysis, the blood sample was obtained through the catheter at the start of treatment, before administration of heparin. For peritoneal dialysis patients, blood was obtained by venipuncture during routine nephrology clinic visits. For control subjects, a sample of blood was collected before a kidney biopsy or during outpatient evaluation of hematuria or proteinuria. A portion of each blood sample (400 μl) was processed immediately to obtain plasma and the remainder (4.6 ml) was used for chemokine receptor profiling (0.6 ml, unfractionated) and functional studies (4 ml, fractionated). For fractionation of neutrophils and mononuclear cells, blood (4 ml) was mixed with 6% T500 dextran (1.4 ml) and allowed to sediment for 20 min. The upper fraction was recovered, and cells were collected by centrifugation, washed, and purified by Ficoll hypaque (Pharmacia; Piscataway, New Jersey) density gradient centrifugation. Cells at the interface were collected and used for monocyte functional studies. Pellets were collected, contaminating red blood cells were removed by hypotonic lysis with water, and the remaining cells were used for neutrophil functional studies. For children on hemodialysis, the effect of the procedure itself was evaluated by obtaining blood samples at the start of hemodialysis and after 60, 120, and 180 min of treatment.
Flow Cytometric Analysis of Chemokine Receptor Expression
To analyze cell surface chemokine receptor expression, EDTA-anticoagulated blood (100 μl per tube) was aliquoted into six tubes containing anti-CD14 APC (for monocyte detection) in 20 μl. Individual tubes then received phycoerythrin (PE)-labeled monoclonal anti-chemokine receptor (CCR1, CCR2, CCR5, CXCR1, or CXCR2) antibody or corresponding isotype control antibody (5 μl of PE-labeled antibody per tube). Reactions were incubated for 20 min, and then erythrocytes were lysed using red blood cell lysis buffer (BD-Pharmingen). Chemokine receptor expression was analyzed by four-color flow cytometry using a Becton Dickinson FACSCalibur. Monocytes were identified by CD14+ staining and neutrophils and lymphocytes were identified by forward and side scatter profile. Results are expressed as the difference between mean fluorescence intensity with the anti-chemokine receptor antibody and isotype control antibody.
Chemotaxis Assays
For neutrophil chemotaxis, purified neutrophils were labeled with 5 μM calcein-AM according to the manufacturer's directions (Molecular Probes, Eugene, Oregon), and diluted to a concentration of 3 × 106 cells per ml in RPMI 1640 medium (Invitrogen) and placed into the upper well of a Transwell chamber that was separated from the bottom well (containing IL-8 chemoattractant) by a membrane with 3-μm pores (Costar). Neutrophils were allowed to migrate for 1 h and then the upper well was removed and neutrophils in the lower well quantified by measuring calcein fluorescence. For monocyte chemotaxis, mononuclear cells were diluted to a concentration of 3 × 106 cells per ml in RPMI 1640 medium (Invitrogen) and placed into the upper well of a Transwell chamber that was separated from the bottom well (containing MCP-1 chemoattractant) by a membrane with 5-μm pores (Costar). Cells were allowed to migrate for 2 h and then the upper well was removed and monocytes in the lower well quantified by staining cells with APC-labeled anti-CD14 antibody, followed by the addition of a fixed amount of FITC-labeled beads (BD Pharmingen) to each tube and quantification of the number of CD14+ cells per 104 beads by flow cytometry.
Chemokine Analyses
Chemokine levels were quantitated in duplicate by specific sandwich ELISA assays. MCP-1, MIP-1α, MIP-1β, and IL-8 concentrations were determined using Quantikine ELISA kits (R&D Systems) and RANTES concentrations were determined using a previously described ELISA assay (19). Absorbances were read using a Dynatech MR5000 spectrophotometer and chemokine concentrations determined by comparison to standard curves using a Biolinx software program.
Statistical Analyses
For plasma chemokines levels and chemokine receptor expression, the statistical analysis began with the use of the Mann–Whitney (MW) nonparametric test to make the simple comparison of control versus ERSD, because that analysis corresponded to the initial research question. Upon finding a significant difference (P < 0.05, with no adjustments made for the testing of multiple variables), the effects of other factors such as infection and type of dialysis were investigated using the Kruskal–Wallis (KW) nonparametric test to compare controls versus ESRD without infection versus ESRD with infection and, similarly, for controls versus peritoneal dialysis (PD) versus hemodialysis (HD). Upon finding a significant difference (P < 0.05), pairwise multiple comparisons using the MW test were carried out to determine which patient groups differed from one another. Because three pairwise tests were performed, a result was considered significant if P < 0.017 (= 0.05/3). One-way ANOVA with Bonferroni post-test was used for the analysis of neutrophil and macrophage migration and a two-way ANOVA was used for analysis of chemokine receptor changes over time during hemodialysis treatment. In these analyses, P < 0.05 was accepted as significant. All statistical analyses were performed using SAS or GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, California).
Because this investigation was a pilot study, no formal power calculations were carried out before conducting the study. Nevertheless, it should be noted that with approximately 16 controls and 30 ESRD patients, the study has 80% power for detecting an effect size of 0.89 between the two groups (alpha = 0.05, 2-tailed t test). Such a difference is often considered “large” in size (20).
Results
Study Population
We studied 30 pediatric patients with ESRD and 16 healthy control children and adolescents. Among the children with ESRD, 21 were being treated with hemodialysis and 9 with peritoneal dialysis. The mean age, gender, and ethnicity of the patients and controls are shown in Table 1. There were no significant differences in any of these characteristics between (i) the two groups of patients with ESRD (HD group and PD group) or (ii) the control group and either ESRD group. The control patients were not taking any medications at the time of blood sampling. Among the ESRD patients, four were prescribed antibiotics—two in the HD group and two in the PD group—but none were being treated with statins.
Table 1.
Clinical characteristics of study population
| Variable | ESRDa/Hemodialysis (n = 21) | ESRDa/ Peritoneal Dialysis (n = 9) | Controls (n = 16) |
|---|---|---|---|
| Age | 13.4 ± 4.6 | 6.2 ± 7.1 | 11.4 ± 6.3 |
| Gender | 13 boys/8 girls | 6 boys/3 girls | 11 boys/5 girls |
| Ethnicity | 6Wb / 13B / 2 Asian | 3W / 3B / 1 Indian / 2 Asian | 8W / 4B / 2 H / 2 Asian |
The underlying causes of ESRD in patient population included: focal segmental glomerulosclerosis (n = 7, associated with cystic fibrosis in 1 case), obstructive uropathy (n = 6), acute tubular necrosis (n = 3), interstitial nephritis (n = 2) membranous nephropathy (n = 2), systemic lupus erythematosus (n = 2), chronic GN (n = 2), autosomal recessive polycystic kidney disease (n = 1), HIV nephropathy (n = 1), poststreptococcal acute FN (n = 1), Alport syndrome (n = 1), microscopic polyangitis (n = 1) and D + HUS (n = 1).
W, white; B, black; H, Hispanic
ESRD is Associated with Dysregulated Expression of a Subset of Chemokine Receptors on Circulating Neutrophils and Monocytes
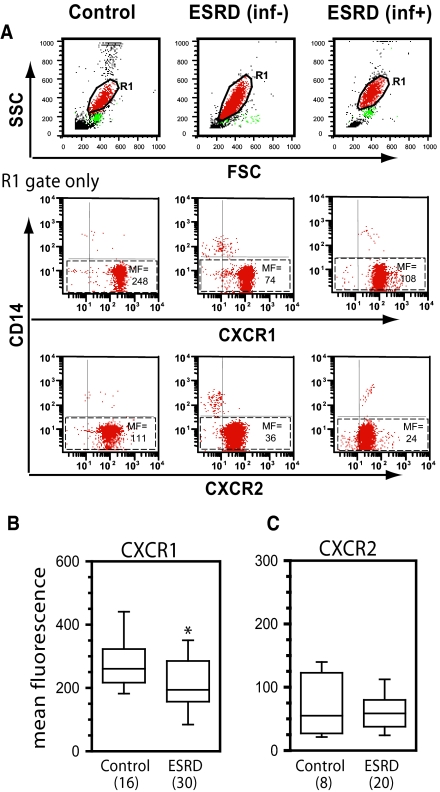
We hypothesized that abnormalities in neutrophil and macrophage function observed within the context of ESRD might result from altered inflammatory cell chemokine receptor expression. To address this possibility, neutrophils and monocytes from patients with ESRD and controls were analyzed for chemokine receptor expression by flow cytometry. Neutrophils from patients with ESRD expressed significantly lower levels of the major neutrophil chemoattractant receptor CXCR1 compared with neutrophils from healthy children and adolescents (P < 0.026) (Figure 1, A and B). Expression of a second neutrophil chemoattractant receptor, CXCR2, was analyzed in a subgroup of study subjects and no significant difference in receptor expression between patients with ESRD and control subjects was observed (Figure 1, A and C). The determination of chemokine receptor levels was repeated in six patients (HD, n = 5 and PD, n = 1) after a 9 to 12-mo observation period and the measurements were reproducible in all instances (data not shown).
Figure 1.
ESRD is associated with dysregulated expression of chemokine receptors on circulating neutrophils. Whole blood was collected and stained for CXCR1 or CXCR2 using phycoerythrin (PE)-conjugated antibodies specific for each receptor. All samples were co-stained with CD14-allophycocyanin (APC) to discriminate neutrophils (CD14−) from contaminating monocytes (CD14+). (A) Representative whole blood forward scatter/side scatter dot plots (to define R1/neutrophil gate) and two-color dot plots (R1/neutrophil gate only) for one healthy control, one patient with ESRD without infection over the preceding 12 mo (inf−), and one patient with ESRD having more than three infections over the preceding 12 mo (inf+). Numbers correspond to mean fluorescence intensity (MF) of chemokine receptor staining in neutrophil population (identified by dashed boxes). (B) Mean neutrophil CXCR1 expression in healthy controls versus patients with ESRD. (C) Mean neutrophil CXCR2 expression in healthy controls versus patients with ESRD. In panels B and C, the numbers in parentheses indicate the number of control or patient samples included in analysis. Where blood was obtained multiple times from a single donor all values were averaged and included as a single value for analysis. Data are plotted in box plot format where the box defines the 25th and 75th percentiles, the line inside the box marks the value of the 50th percentile, and the capped bars indicate the 10th and 90th percentiles. Data were obtained and analyzed as described in the Statistical Analyses section. The symbol * indicates P < 0.026 versus control.
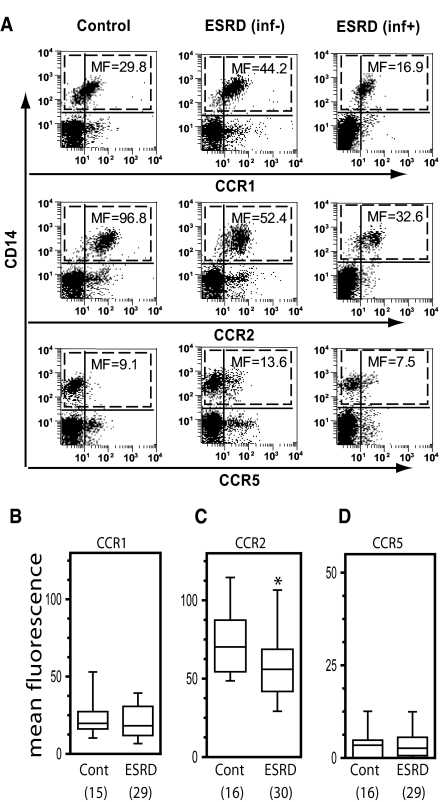
Circulating monocytes display a different pattern of chemokine receptors on their surface as compared with neutrophils, allowing for selectivity in their recruitment and activation (21,22). The predominant cell surface chemokine receptor expressed on circulating monocytes is CCR2, which binds MCP-1 (23). Monocytes also express CCR1 (which binds MIP-1α and RANTES), and very low levels of CCR5 (which binds MIP-1α, MIP-1β, and RANTES) (22). Monocytes from ESRD patients and healthy controls were independently analyzed for expression of CCR1, CCR2, and CCR5 by flow cytometry. In the case of these three receptors, a significant difference was observed between the groups in CCR2 receptor expression (P < 0.033), but not CCR1 or CCR5 (Figure 2, A through D). As outlined in the Methods, further subgroup analyses were performed only for the two receptors, CXCR1 and CCR2, which were noted to be different in ESRD patients compared with the controls.
Figure 2.
Cell surface expression of the macrophage chemokine receptor CCR2, but not CCR1 or CCR5, is significantly lower on blood monocytes from patients with ESRD as compared with blood monocytes from control individuals. Whole blood was collected and stained for CCR1, CCR2 or CCR5 using PE-conjugated antibodies specific for each receptor. All samples were co-stained with CD14-APC to identify monocytes (CD14+). (A) Representative whole blood two-color dot plots for one healthy control, one patient with ESRD (inf−), and one patient with ESRD (inf+). Numbers correspond to MF of chemokine receptor staining in CD14+ monocyte population (identified by dashed boxes). (B through D) Mean monocyte CCR1 (panel B), CCR2 (panel C), and CCR5 (panel D) expression in healthy controls versus patients with ESRD. Data were obtained and analyzed as described in the Statistical Analyses section. The symbol * indicates P < 0.033 versus control.
Chemokine Receptor Impairment is Correlated with Infection Status
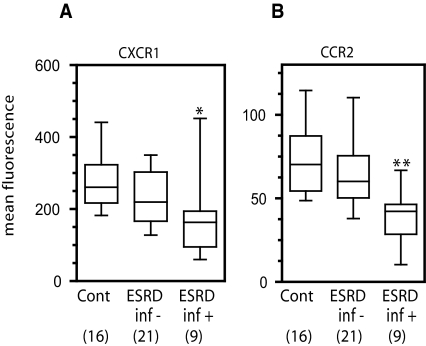
We hypothesized that the reduction in the expression of CXCR1 on neutrophils and CCR2 on monocytes might be greater in the subset of patients with ESRD and recurrent SBI. To address this, the levels of neutrophil CXCR1 (Figure 3A) and monocyte CCR2 (Figure 3B) were analyzed as a function of infection status (controls versus ESRD without infection versus ESRD with infection). Significant differences between groups were found for both CXCR1 (P < 0.014, KW test) and CCR2 (P < 0.011, KW test). Upon further analysis using multiple pairwise comparisons (MW test), controls were found to differ from ESRD with infection for CXCR1 (P < 0.0042) and CCR2 (P < 0.0035). No other comparisons (e.g. controls versus ESRD without infection or ESRD with infection versus ESRD without infection) were statistically significant (Table 2), although patients with ESRD who did not experience recurrent infection had a trend toward lower CXCR1 expression compared with controls that was not statistically significant.
Figure 3.
Infection status regulates chemokine receptor expression. Whole blood was collected and screened for CXCR1 (panel A) or CCR2 (panel B) by staining with PE-conjugated antibodies specific for each receptor and then analyzing by flow cytometry gating on neutrophils (panel A) or CD14+ monocytes (panel B). Patients were divided into those without infection over the preceding 12 mo (inf−) and those having more than three infections over the preceding 12 mo (inf+). Subgroup analyses were performed using the Kruskal–Wallis nonparametric test followed by pairwise multiple comparisons using the Mann–Whitney test as described in the Statistical Analyses section. Significances are indicated as (*) P < 0.0042 versus control, (**) P < 0.0035 versus control.
Table 2.
Chemokine receptor expression levelsa
| Chemokine Receptor | Controls | ESRD | ESRD No Infection | ESRD Recurrent Infection | ESRD Hemodialysis | ESRD Peritoneal Dialysis |
|---|---|---|---|---|---|---|
| CCR1 | 23.9 ± 3.6 (15) | 21.1 ± 2.3 (29) | 22.2 ± 2.7 (20) | 18.5 ± 4.4 (9) | 22.1 ± 2.8 (20) | 18.7 ± 4.0 (9) |
| CCR2 | 74.7 ± 6.1 (16) | 61.6 ± 6.1 (30)b | 68.8 ± 7.7 (21) | 44.5 ± 7.7 (9)b | 63.1 ± 8.1 (21) | 58.0 ± 8.4 (9) |
| CCR5 | 4.0 ± 1.1 (16) | 3.5 ± 0.7 (29) | 2.2 ± 0.5 (20) | 6.5 ± 1.5 (9) | 3.8 ± 0.8 (20) | 2.9 ± 1.31 (9) |
| CXCR1 | 276.4 ± 21.4 (16) | 214.8 ± 18.3 (30)b | 233.1 ± 18.9 (21) | 172.1 ± 38.8 (9)b | 209.0 ± 21.8 (21) | 228.4 ± 35.3 (9) |
| CXCR2 | 69.1 ± 17.76 (8) | 61.3 ± 6.7 (20) | 59.0 ± 4.0 (15) | 68.2 ± 21.1 (5) | 63.5 ± 9.8 (12) | 58.0 ± 8.8 (8) |
Mean ± SEM from indicated number of control subjects or patients (number of control subjects or patients in parentheses). Chemokine receptor expression values are reported as mean fluorescence intensity. Significances for control versus ESRD were determined using the Mann–Whitney nonparametric test. Significances for all subgroup analyses were determined using the Kruskal–Wallis nonparametric test followed by pairwise multiple comparisons using the Mann–Whitney test.
P < 0.017 versus control.
A Comparable Pattern of Chemokine Receptor Expression is Observed in Patients on HD and PD
To assess whether chemokine receptor expression might be differentially regulated in patients on HD (n = 21) versus those on PD (n = 9), we analyzed cell surface chemokine receptor levels on both neutrophils and monocytes as a function of dialysis modality. Neither CXCR1 levels on neutrophils nor CCR2 levels on monocytes differed significantly between the two renal replacement therapy groups (Table 2).
There is a Time-Dependent Loss of Chemokine Receptor Expression as a Function of the HD Process Itself
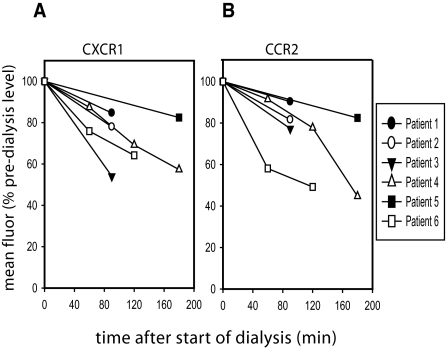
In the studies outlined above, blood was drawn from HD patients immediately before the initiation of dialysis. Any chemokine receptor loss therefore reflects dysregulation in baseline CXCR1 and CCR2 expression. Because components of the dialysis membrane have an effect on cellular activation (24,25), we also explored whether the HD procedure itself had an adverse effect on chemokine receptor expression. In a subset of patients (n = 6), a kinetic study was performed in which blood was collected before HD and at defined intervals after the initiation of the procedure. We observed CXCR1 loss on neutrophils (Figure 4A) and CCR2 loss on monocytes (Figure 4B) as a function of duration of HD. The decline in chemokine receptor expression was observed in all patients in this subgroup irrespective of infection status, and was confirmed on repeat testing in two children.
Figure 4.
There is a time-dependent loss of chemokine receptor expression as a function of the dialysis process itself. The effect of the dialysis procedure itself on neutrophil CXCR1 expression (Figure 4A) and CCR2 on circulating monocytes (Figure 4B) was evaluated in a subset of hemodialysis patients by collecting EDTA anticoagulated blood at the start of hemodialysis and at specified time-points after initiation of treatment and analyzing CXCR1 expression by flow cytometry. Data from six independent patients are plotted here. For five patients for whom 60 to 90-min time point data were available, we compared those values to predialysis values using two-way ANOVA and found the percent decreases in CXCR1 and CCR2 expression to be significantly different as a function of time (CXCR1: P < 0.01; CCR2: P < 0.03), but not as a function of patient (P > 0.5 for both).
Chemokine Receptor Function is Impaired in ESRD
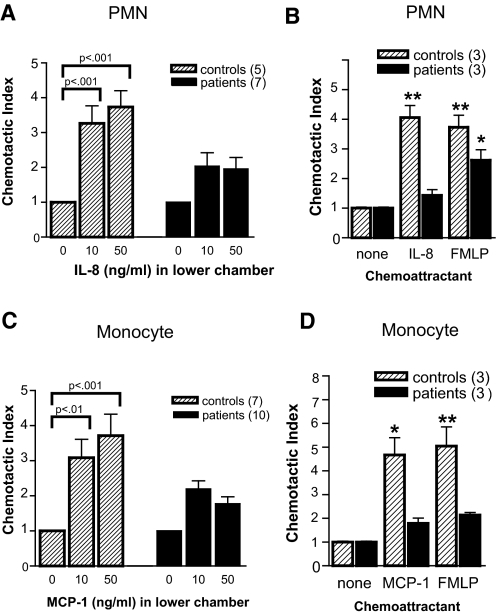
Although chemokine receptor expression was significantly lower in both neutrophils (CXCR1) and monocytes (CCR2) from patients with ESRD, it was never lost completely. To address whether the low levels of immunoreactive receptor present on ESRD patient leukocytes were functional, we independently isolated neutrophils and monocytes and assayed their responsiveness to the appropriate chemokine ligands (IL-8 for neutrophils, MCP-1 for monocytes) using a modified Boyden chamber chemotaxis assay. These studies were done in nine ESRD patients, of whom three had recurrent SBI and six were infection-free. Although neutrophils isolated from healthy control children and adolescents exhibited strong migratory responses to the CXCR1/CXCR2 ligand IL-8 (Figure 5A, hatched bars), neutrophils isolated from ESRD patients exhibited impaired responsiveness to this chemoattractant (Figure 5A, solid bars). Similarly, monocytes isolated from ESRD patients exhibited markedly lower responses to the CCR2 ligand MCP-1 as compared with monocytes isolated from healthy control children (Figure 5C). To address whether the cells from children with ESRD have a specific defect in chemokine receptor trafficking or a global defect in migration, we tested their responsiveness to N-formyl-methionyl-leucyl-phenylalanine (FMLP), a potent chemoattractant for both neutrophils and monocytes that acts independently of the chemokine receptors. Neutrophils from children with ESRD, although unresponsive to IL-8, were able to migrate in response to FMLP (Figure 5B). In contrast, monocytes from patients with ESRD exhibited impaired migration to both MCP-1 and FMLP (Figure 5D), suggesting a more global defect in trafficking in these cells. Interestingly, these later studies were performed in the three patients without recurrent SBI.
Figure 5.
Chemokine receptor function is impaired in ESRD. (A and B) Neutrophils were fluorescently labeled and assayed for responsiveness to IL-8 and N-formyl-methionyl-leucyl-phenylalanine (FMLP) in transwell chemotaxis assays. Data are reported as Chemotactic Index (fluorescenceEXP/fluorescenceCONTROL). (C and D) Mononuclear cells were assayed for responsiveness to monocyte chemotactic protein-1 (MCP-1)/CCL2 and FMLP in transwell chemotaxis assays. Data are reported as Chemotactic Index (# CD14+ cellsEXP/# CD14+ cellsCONTROL. In panels A and C, bars represent the means ± SD of all controls and patients analyzed and the numbers on the x-axis indicate the concentration of IL-8 or MCP-1/CCL2 in the bottom well of the chemotaxis chamber, respectively. In panels B and D, bars represent the means ± SD of three control and three patients analyzed, and chemoattractant concentrations were IL-8 (50 ng/ml) and FMLP (10−8M). All chemotaxis assays were performed in duplicate. Statistical analysis comparing migration in response to chemoattractants to migration in the absence of any chemoattractant was performed using one-way ANOVA with Bonferroni post test.
Circulating Chemokines are Elevated in Patients with ESRD, but the Levels Do Not Correlate with Chemokine Receptor Dysregulation.
A subset of chemokine ligands, including those that regulate the trafficking of circulating monocytes and neutrophils (MCP-1, MIP-1α, MIP-1β, RANTES, and IL-8) are upregulated within the context of renal disease (26–29). Therefore, we measured plasma levels of those five chemokines in our cohort of children with ESRD and healthy controls (Table 3). Controls only differed significantly from ESRD patients for MCP-1 (P < 0.0004). No differences were observed for MIP-1α, MIP-1β, RANTES, or IL-8. When the effect of type of dialysis was used to further stratify the ESRD patients (PD versus HD), it was observed that MCP-1 differed for control versus PD and for control versus HD, but not for PD versus HD.
Table 3.
Plasma chemokine levelsa
| Chemokine | Controls (n = 12) | ESRD (n = 28) | ESRD No Infection (n = 19) | ESRD Recurrent Infection (n = 9) | ESRD Hemodialysis (n = 19) | ESRD Peritoneal Dialysis (n = 9) |
|---|---|---|---|---|---|---|
| MCP-1 | 153 ± 29 | 389 ± 40b | 335 ± 44b | 504 ± 72b | 372 ± 46b | 427 ± 80b |
| MIP-1α | 236 ± 60 | 146 ± 7 | 142 ± 6 | 154 ± 17 | 143 ± 9 | 153 ± 7 |
| MIP-1β | 186 ± 32 | 124 ± 8 | 116 ± 6 | 141 ± 21 | 127 ± 11 | 118 ± 101 |
| RANTES | 1450 ± 225 | 1002 ± 84 | 1003 ± 92 | 999 ± 133 | 1025 ± 102 | 953 ± 157 |
| IL-8 | 658 ± 135 | 414 ± 34 | 366 ± 25 | 516 ± 86 | 411 ± 46 | 420 ± 47 |
Mean values ± SEM from indicated number of control subjects or patients. Chemokine values are reported in pg/ml. Specimens with undetectable chemokine concentrations were assigned a value equal to the lower limit of detection. Significances for control versus ESRD were determined using the Mann–Whitney nonparametric test. Significances for all subgroup analyses were determined using the Kruskal–Wallis nonparametric test followed by pairwise multiple comparisons using the Mann–Whitney test.
P < 0.017 versus control.
MCP-1, monocyte chemotactic protein-1; MIP-1α, macrophage inflammatory protein-1α; MIP-1β, macrophage inflammatory protein-1β; RANTES, regulated upon activation, normal T cell expressed, and secreted protein; IL-8, interleukin-8.
When stratified according to infection status, there was a significant difference for MCP-1 (P < 0.0003, KW test). Upon further analysis with multiple comparisons, controls were found to differ from ESRD with infection and from ESRD without infection. However, ESRD with infection did not differ from ESRD without infection. There was no correlation between plasma chemokine levels and the level of chemokine receptor expression on leukocytes in either control subjects or patients with ESRD.
Discussion
Cellular responses to chemokine ligands, which are essential for mounting an effective host defense against pathogens, are critically dependent upon the expression of functional chemokine receptors on the surface of inflammatory cells. Our data demonstrate a marked dysregulation in inflammatory cell chemokine receptor expression and responsiveness in pediatric patients with ESRD, which is more pronounced in the subgroup of patients who have had multiple SBI in the preceding year. The fact that impairment in cell surface chemokine receptor expression on circulating neutrophils and monocytes correlates with recurrent infection suggests that chemokine receptor dysregulation might underlie or contribute to an increased risk for infection, and as such, may be a useful index of treatment efficacy.
It is important to acknowledge that our findings were obtained in a modest cohort of pediatric patients and age-matched controls recruited at a single site. Patient cohorts for studies in children with ESRD must be evaluated in light of the lower incidence of disease and the impetus to kidney transplantation in pediatric versus adult populations. Nonetheless, valid conclusions about immune function, e.g. plasma cytokine levels have been drawn based on patients groups half as large as ours (30). The results of our study need to be confirmed in adults with ESRD. In addition, the study group had a wide variety of underlying kidney diseases and because of the limited sample size we cannot make any assertion about the effects of specific kidney disorders on chemokine receptor levels and circulating chemokine concentrations. However, we observed alterations in chemokine receptor levels in patients with glomerular or tubulointerstitial disease, suggesting that the dysregulation is an intrinsic feature of ESRD.
The differences in chemokine receptor expression levels observed in our pediatric populations do not appear to be attributable to age, as there was no significant difference in age between (i) the two groups of patients with ESRD (HD group and PD group) or (ii) the control group and either ESRD group. A recent report has documented a difference in postinfection CCR1 and CCR5 expression on influenza-infected monocyte-derived macrophages from human umbilical cord blood of healthy full-term infants as compared with influenza-infected monocyte-derived macrophages from adult blood donors (31). However, that study focused on postinfection differences in neonatal versus adult chemokine receptor expression in cultured macrophages and did not evaluate basal expression of chemokine receptors in unstimulated peripheral blood monocytes and neutrophils, as in our study. Moreover, the pediatric patients and controls in our cohort were beyond the newborn period. Finally, there was no correlation between age and chemokine receptor expression levels in our control population (age range 3 mo to 20.7 yr; r2 = 0.041; P = 0.51 for CXCR1 expression on neutrophils; r2 = 0.079 with P = 0.35 for CCR2 expression on monocytes).
Chemokine receptor expression and responsiveness is dysregulated in both neutrophils and monocytes in the context of ESRD. Moreover, the two cell types are similarly responsive to disease conditioning. The levels of the neutrophil chemoattractant receptor, CXCR1, and the monocyte chemoattractant receptor, CCR2, are both mildly diminished as a consequence of ESRD itself. Recurrent SBI markedly exacerbated this loss on both cell types. Therefore, in patients with ESRD, changes in neutrophil CXCR1 and monocyte CCR2 expression as a reflection of a fixed abnormality inherent to the uremic state may be less prominent compared with the alterations induced by recurrent SBI.
We acknowledge that our studies do not define the cellular basis or underlying mechanism for the observed alteration in chemokine receptor expression. Changes in leukocyte adhesion molecule expression or membrane microvilli may play key roles in this process. Another potential mechanism that may contribute to the chemokine receptor dysregulation in ESRD is receptor crossdesensitization. In neutrophils, calcium mobilization triggered by CXCR1 and CXCR2 ligands (e.g. IL-8 and groα) is desensitized by prior exposure to several unrelated chemoattractants including N-formylated peptides and the complement cleavage product C5a (32,33). Circulating levels of complement components C5a and C3a are elevated in patients with ESRD on dialysis (34,35), and chronic exposure of circulating inflammatory cells to these mediators may lead to loss of chemokine receptor expression and/or function via crossdesensitization. This highlights the dual nature of complement, which plays a protective role against bacterial infection on the one hand, but on the other can elicit an exaggerated inflammatory response that contributes to disease-related pathology (36–39). Although it is likely that complement components contribute to this phenomenon, it is unlikely to be the sole factor. First, the effect of C5a is much more dramatic on CXCR2 as opposed to CXCR1, whereas herein we observed significantly lower expression of CXCR1, but not CXCR2, on neutrophils from patients with ESRD. Second, although complement components exert similar effects on the β chemokine receptor, CCR2, our data only show a significant reduction in CCR2 on monocytes within the context of infection and not in patients with ESRD without active infection, even though the levels of complement components would be similar in both groups. Studies to elucidate the mechanism of chemokine dysregulation in children with ESRD are underway in our laboratory.
Our results underscore the disparity between chemokine receptor expression and function. Although levels of inflammatory chemokine receptors are significantly lower on both neutrophils and monocytes from patients with ESRD who had three or more SBI in the preceding year as compared with healthy controls, they are still detectable. Only when receptor function is evaluated does the full effect of chemokine receptor impairment become apparent, with monocytes and neutrophils being almost completely unresponsive to chemokine ligands. This may reflect the need for a threshold of receptor expression for cells to respond to chemokine ligands. Alternatively, some of the receptor expressed on the cell surface may be immunoreactive, but biologically inactive. Finally, uremia may interfere with downstream signaling after ligand binding to the appropriate chemokine receptor that impairs normal cell function. Therefore, even cells with detectable levels of chemokine receptors may be incapable of migration in response to ligand. This would imply that measuring chemokine receptor levels underestimates chemokine receptor defects, and that expression studies need to be complemented with functional assays.
A critical question is the identity of the initial stimulus that triggers the downregulation of chemokine receptors. Does early exposure to SBI result in loss of cell surface chemokine receptors, impairing the ability to fight subsequent infections, or are chemokine receptors downmodulated as a consequence of renal disease, resulting in increased susceptibility to bacterial infection? It is difficult to distinguish between these two possibilities on the basis of our data, but both processes are likely to be involved. On the one hand, bacterial products including lipopolysacharides can downregulate chemokine receptor expression on several cell types critical for innate immune responses, including neutrophils and macrophages (40–44). On the other hand, failure to express functional chemokine receptors in targeted gene knockout studies in mice and single nucleotide polymorphism analysis in humans leads to heightened risk of bacterial infection (11–18,45). Thus, the following sequence of steps may occur in ESRD: primary reduction in neutrophil and monocyte chemokine receptor levels, increased susceptibility to infection, further lowering of neutrophil and macrophage chemokine receptor levels, and further increased susceptibility to infection. The demonstration of two different subpopulations of patients, one that experienced recurrent SBI and the other that did not, may reflect a recent infection that acutely lowered chemokine responsiveness in the former group. Another possibility is that there are inherent genetic differences, reflected in single nucleotide polymorphism variability, in chemokine expression between the two groups. Finally, environmental or treatment factors may contribute to the variability in chemokine receptor expression in pediatric ESRD patients.
A recent study reported elevated expression of CXCR1 by neutrophils in the glomerular and tubulointerstitial renal compartments in patients with lupus nephritis, membranoproliferative GN, and crescentic GN (46). This suggests that the loss of chemokine receptor expression on leukocytes is restricted to the blood compartment. This dichotomy may lead to enhanced intrarenal inflammation-induced pathology in these diseases, in conjunction with decreased inflammatory responses to pathogens. It raises concern regarding the use of small molecule anti-chemokine inhibitors to block chemokine-mediated pathology in the kidney (47,48) because they could increase the risk of SBI in patients with ESRD.
In summary, we have demonstrated that chemokine receptor dysregulation contributes to the immune dysfunction that is involved in the development of catheter-related SBI in children with ESRD. Moreover, the findings raise the possibility that analysis of chemokine receptor levels on circulating leukocytes may identify a subset of patients on dialysis who are at increased risk of developing SBI
Disclosures
None.
Acknowledgments
The authors are grateful for the contributions of Rachel Frank, RN from Schneider Children's Hospital and Sharyn Parness, RN and Erica Christen, RN from The Feinstein Institute for Medical Research GCRC in helping to coordinate blood collection from study subjects. This work was supported by NIH R01 AI059742 (BAS), NIH DK70341 (HT), and a grant to The Feinstein Institute for Medical Research GCRC (GCRC M01 RR018535). This work was presented in abstract form at CR2004, Chicago, Illinois (April 2004).
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Marr KA, Kong L, Fowler VG, Gopal A, Sexton DJ, Conlon PJ, Corey GR: Incidence and outcome of Staphylococcus aureus bacteremia in hemodialysis patients. Kidney Int 54: 1684–1689, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Liangos O, Gul A, Madias NE, Jaber BL: Long-term management of the tunneled venous catheter. Semin Dial 19: 158–164, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Beathard GA: Management of bacteremia associated with tunneled-cuffed hemodialysis catheters. J Am Soc Nephrol 10: 1045–1049, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Tanriover B, Carlton D, Saddekni S, Hamrick K, Oser R, Westfall AO, Allon M: Bacteremia associated with tunneled dialysis catheters: Comparison of two treatment strategies. Kidney Int 57: 2151–2155, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Bunke CM, Brier ME, Golper TA: Outcomes of single organism peritonitis in peritoneal dialysis: Gram negatives versus gram positives in the Network 9 Peritonitis Study. Kidney Int 52: 524–529, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Port FK, Held PJ, Nolph KD, Turenne MN, Wolfe RA: Risk of peritonitis and technique failure by CAPD connection technique: A national study. Kidney Int 42: 967–974, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Ruiz P, Gomez F, Schreiber AD: Impaired function of macrophage Fc gamma receptors in end-stage renal disease. N Engl J Med 322: 717–722, 1990 [DOI] [PubMed] [Google Scholar]
- 8.Baggiolini M, Dewald B, Moser B: Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol 55: 97–179, 1994 [PubMed] [Google Scholar]
- 9.Adrie C, Pinsky MR: The inflammatory balance in human sepsis. Intensive Care Med 26: 364–375, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Wang JM, Sherry B, Fivash MJ, Kelvin DJ, Oppenheim JJ: Human recombinant macrophage inflammatory protein-1 alpha and -beta and monocyte chemotactic and activating factor utilize common and unique receptors on human monocytes. J Immunol 150: 3022–3029, 1993 [PubMed] [Google Scholar]
- 11.Maus U, von Grote K, Kuziel WA, Mack M, Miller EJ, Cihak J, Stangassinger M, Maus R, Schlondorff D, Seeger W, Lohmeyer J: The role of CC chemokine receptor 2 in alveolar monocyte and neutrophil immigration in intact mice. Am J Respir Crit Care Med 166: 268–273, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Kurihara T, Warr G, Loy J, Bravo R: Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med 186: 1757–1762, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Souza CD, Cooper AM, Frank AA, Ehlers S, Turner J, Bendelac A, Orme IM: A novel nonclassic beta2-microglobulin-restricted mechanism influencing early lymphocyte accumulation and subsequent resistance to tuberculosis in the lung. Am J Respir Cell Mol Biol 23: 188–193, 2000 [DOI] [PubMed] [Google Scholar]
- 14.DiTirro J, Rhoades ER, Roberts AD, Burke JM, Mukasa A, Cooper AM, Frank AA, Born WK, Orme IM: Disruption of the cellular inflammatory response to Listeria monocytogenes infection in mice with disruptions in targeted genes. Infect Immun 66: 2284–2289, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindell DM, Standiford TJ, Mancuso P, Leshen ZJ, Huffnagle GB: Macrophage inflammatory protein 1alpha/CCL3 is required for clearance of an acute Klebsiella pneumoniae pulmonary infection. Infect Immun 69: 6364–6369, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao JL, Wynn TA, Chang Y, Lee EJ, Broxmeyer HE, Cooper S, Tiffany HL, Westphal H, Kwon-Chung J, Murphy PM: Impaired host defense, hematopoiesis, granulomatous inflammation and type 1-type 2 cytokine balance in mice lacking CC chemokine receptor 1. J Exp Med 185: 1959–1968, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olszewski MA, Huffnagle GB, McDonald RA, Lindell DM, Moore BB, Cook DN, Toews GB: The role of macrophage inflammatory protein-1 alpha/CCL3 in regulation of T cell-mediated immunity to Cryptococcus neoformans infection. J Immunol 165: 6429–6436, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Zeng X, Moore TA, Newstead MW, Hernandez-Alcoceba R, Tsai WC, Standiford TJ: Intrapulmonary expression of macrophage inflammatory protein 1alpha (CCL3) induces neutrophil and NK cell accumulation and stimulates innate immunity in murine bacterial pneumonia. Infect Immun 71: 1306–1315, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidtmayerova H, Nottet HS, Nuovo G, Raabe T, Flanagan CR, Dubrovsky L, Gendelman HE, Cerami A, Bukrinsky M, Sherry B: Human immunodeficiency virus type 1 infection alters chemokine beta peptide expression in human monocytes: implications for recruitment of leukocytes into brain and lymph nodes. Proc Natl Acad Sci USA 93: 700–704, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen J: The T-test for means, F-tests on means in the analysis of variance and covariance. In: Statistical Power Analysis for the Behavioral Sciences, New York, Academic Press, 1997
- 21.Mantovani A, Muzio M, Garlanda C, Sozzani S, Allavena P: Macrophage control of inflammation: Negative pathways of regulation of inflammatory cytokines. Novartis Found Symp 234: 120–131; discussion 131–135: 120–131, 2001 [DOI] [PubMed]
- 22.Moser B, Wolf M, Walz A, Loetscher P: Chemokines: Multiple levels of leukocyte migration control. Trends Immunol 25: 75–84, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Maus UA, Waelsch K, Kuziel WA, Delbeck T, Mack M, Blackwell TS, Christman JW, Schlondorff D, Seeger W, Lohmeyer J: Monocytes are potent facilitators of alveolar neutrophil emigration during lung inflammation: Role of the CCL2-CCR2 axis. J Immunol 170: 3273–3278, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Gesualdo L, Pertosa G, Grandaliano G, Schena FP: Cytokines and bioincompatibility. Nephrol Dial Transplant 13: 1622–1626, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Horl WH: Hemodialysis membranes: Interleukins, biocompatibility, and middle molecules. J Am Soc Nephrol 13 [Suppl 1]: S62–S71, 2002 [PubMed] [Google Scholar]
- 26.Kelley VR, Rovin BH: Chemokines: Therapeutic targets for autoimmune and inflammatory renal disease. Springer Semin Immunopathol 24: 411–421, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Segerer S: The role of chemokines and chemokine receptors in progressive renal diseases. Am J Kidney Dis 41: S15–S18, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Anders HJ, Vielhauer V, Schlondorff D: Chemokines and chemokine receptors are involved in the resolution or progression of renal disease. Kidney Int 63: 401–415, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Lin CY, Lin CC, Huang TP: Serial changes of interleukin-6 and interleukin-8 levels in drain dialysate of uremic patients with continuous ambulatory peritoneal dialysis during peritonitis. Nephron 63: 404–408, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Goldstein SL, Leung JC, Silverstein DM: Pro- and anti-inflammatory cytokines in chronic pediatric dialysis patients: Effect of aspirin. Clin J Am Soc Nephrol 1: 979–986, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Zhou J, Law HK, Cheung CY, Ng IH, Peiris JS, Lau YL: Differential expression of chemokines and their receptors in adult and neonatal macrophages infected with human or avian influenza viruses. J Infect Dis 194: 61–70, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson RM, Pridgen BC, Haribabu B, Ali H, Snyderman R: Differential cross-regulation of the human chemokine receptors CXCR1 and CXCR2. Evidence for time-dependent signal generation. J Biol Chem 273: 23830–23836, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Sabroe I, Williams TJ, Hebert CA, Collins PD: Chemoattractant cross-desensitization of the human neutrophil IL-8 receptor involves receptor internalization and differential receptor subtype regulation. J Immunol 158: 1361–1369, 1997 [PubMed] [Google Scholar]
- 34.Miyata T, Inagi R, Hong K, Iida Y, Oda O, Kinoshita T, Inoue K, Miyama A, Maeda K: Fluid-phase activation of the alternative pathway of complement by excess factor D in regularly dialyzed patients. Nephron 60: 144–149, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Deppisch RM, Beck W, Goehl H, Ritz E: Complement components as uremic toxins and their potential role as mediators of microinflammation. Kidney Int [Suppl 78]: S271–S277, 2001 [DOI] [PubMed]
- 36.Amore A, Coppo R: Immunological basis of inflammation in dialysis. Nephrol Dial Transplant 17 [Suppl 8]: 16–24, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Cunningham PN, Quigg RJ: Contrasting roles of complement activation and its regulation in membranous nephropathy. J Am Soc Nephrol 16: 1214–1222, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Helmy KY, Katschke KJ Jr, Gorgani NN, Kljavin NM, Elliott JM, Diehl L, Scales SJ, Ghilardi N, van Lookeren CM: CRIg: A macrophage complement receptor required for phagocytosis of circulating pathogens. Cell 124: 915–927, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Allegretti M, Moriconi A, Beccari AR, Di Bitondo R, Bizzarri C, Bertini R, Colotta F: Targeting C5a: Recent advances in drug discovery. Curr Med Chem 12: 217–236, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Khandaker MH, Xu L, Rahimpour R, Mitchell G, De Vries ME, Pickering JG, Singhal SK, Feldman RD, Kelvin DJ: CXCR1 and CXCR2 are rapidly down-modulated by bacterial endotoxin through a unique agonist-independent, tyrosine kinase-dependent mechanism. J Immunol 161: 1930–1938, 1998 [PubMed] [Google Scholar]
- 41.Khandaker MH, Mitchell G, Xu L, Andrews JD, Singh R, Leung H, Madrenas J, Ferguson SS, Feldman RD, Kelvin DJ: Metalloproteinases are involved in lipopolysaccharide- and tumor necrosis factor-alpha-mediated regulation of CXCR1 and CXCR2 chemokine receptor expression. Blood 93: 2173–2185, 1999 [PubMed] [Google Scholar]
- 42.Sabroe I, Jones EC, Whyte MK, Dower SK: Regulation of human neutrophil chemokine receptor expression and function by activation of Toll-like receptors 2 and 4. Immunology 115: 90–98, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franchin G, Zybarth G, Dai WW, Dubrovsky L, Reiling N, Schmidtmayerova H, Bukrinsky M, Sherry B: Lipopolysaccharide inhibits HIV-1 infection of monocyte-derived macrophages through direct and sustained down-regulation of CC chemokine receptor 5. J Immunol 164: 2592–2601, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Parker LC, Whyte MK, Vogel SN, Dower SK, Sabroe I: Toll-like receptor (TLR)2 and TLR4 agonists regulate CCR expression in human monocytic cells. J Immunol 172: 4977–4986, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Ianni A, Majore S, Arzani D, Carboni I, Corbo GM, Romano-Spica V: CCR2 and CCR5 gene polymorphisms in children with recurrent respiratory infections. Respir Med 95: 430–432, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Segerer S, Henger A, Schmid H, Kretzler M, Draganovici D, Brandt U, Noessner E, Nelson PJ, Kerjaschki D, Schlondorff D, Regele H: Expression of the chemokine receptor CXCR1 in human glomerular diseases. Kidney Int 69: 1765–1773, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Rovin BH: Chemokine blockade as a therapy for renal disease. Curr Opin Nephrol Hypertens 9: 225–232, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Segerer S, Alpers CE: Chemokines and chemokine receptors in renal pathology. Curr Opin Nephrol Hypertens 12: 243–249, 2003 [DOI] [PubMed] [Google Scholar]