Abstract
Background and objectives: Secondary analysis of the Hemodialysis Study showed that serum β2-microglobulin levels predicted all-cause mortality and that high-flux dialysis was associated with decreased cardiac deaths in hemodialysis patients. This study examined the association of serum β2-microglobulin levels and dialyzer β2-microglobulin kinetics with the two most common causes of deaths: Cardiac and infectious diseases.
Cox regression analyses were performed to relate cardiac or infectious deaths to cumulative mean follow-up predialysis serum β2-microglobulin levels while controlling for baseline demographics, comorbidity, residual kidney function, and dialysis-related variables.
Results: The cohort of 1813 patients experienced 180 infectious deaths and 315 cardiac deaths. The adjusted hazard ratio for infectious death was 1.21 (95% confidence interval 1.07 to 1.37) per 10-mg/L increase in β2-microglobulin. This association was independent of the prestudy years on dialysis. In contrast, the association between serum β2-microglobulin level and cardiac death was not statistically significant. In similar regression models, higher cumulative mean Kt/V of β2-microglobulin was not significantly associated with either infectious or cardiac mortality in the full cohort but exhibited trends suggesting an association with lower infectious mortality (relative risk 0.93; 95% confidence interval 0.86 to 1.01, for each 0.1-U increase in β2-microglobulin Kt/V) and lower cardiac mortality (relative risk 0.93; 95% confidence interval 0.87 to 1.00) in the subgroup with >3.7 prestudy years of dialysis.
Conclusions: These results generally support the notion that middle molecules are associated with systemic toxicity and that their accumulation predisposes dialysis patients to infectious deaths, independent of the duration of maintenance dialysis.
The Hemodialysis (HEMO) Study was a randomized clinical trial designed to examine the impact of two treatment parameters on clinical outcomes of maintenance hemodialysis patients. These parameters were the dialysis dosage based on the clearance of urea (molecular weight [MW] 60 Da) and membrane porosity or flux based on the clearance of β2-microglobulin (β2M; MW 11,800 Da) (1). The primary analysis of the HEMO Study did not show a statistically significant reduction in the rate of the primary outcome, all-cause mortality, or any of the predefined secondary outcomes associated with high flux. In secondary analyses, however, a 20% decrease in cardiac death (hazard ratio [HR] 0.80; 95% confidence interval [CI] 0.65 to 0.99) was observed for the high-flux group compared with the low-flux group. In the subgroup of patients who had been on dialysis for >3.7 yr (the mean for the entire cohort) before enrollment in the HEMO Study, high flux was associated with lower all-cause mortality (HR 0.68; 95% CI 0.53 to 0.86), cardiac deaths (HR 0.63; 95% CI 0.43 to 0.92) (1,2), and cerebrovascular events (3).
Although these are results of secondary analyses and must be interpreted cautiously because of the multiple hypotheses that were tested (4), they are consistent with the notion that high-flux dialysis may have certain beneficial effects. In the HEMO Study, membrane flux was defined by the clearance of β2M, taken as a surrogate for the clearance of middle molecules. As a result of the higher clearance, the cumulative mean predialysis serum β2M level during follow-up in the high-flux arm was statistically significantly lower than that in the low-flux arms (33.6 versus 41.5 mg/L) (5). Further secondary analysis of the data showed that predialysis serum β2M levels predicted all-cause mortality in the HEMO Study cohort, with an 11% increase in mortality for each 10-mg/L increase in β2M level, even after adjustment for years on dialysis and residual kidney function (5). The specific causes of death that account for this increased mortality have not been determined.
In addition to a number of other middle molecules, in vitro studies have identified proteins from the circulating plasma of maintenance dialysis patients, with homology or MW similar to β2M, that have neutrophil-inhibitory effects (6,7). The accumulation of these proteins leads to higher serum β2M concentrations and may predispose the patients to infectious complications. Furthermore, because randomization to high flux was associated with a decrease in cardiac deaths in the HEMO Study and an even greater reduction in patients who were on dialysis for >3.7 yr before the study (1,2), it would be reasonable to hypothesize that serum β2M levels are also predictive of cardiac death, especially in the long-term dialysis subgroup. In this report, we examined the association of serum β2M levels and dialyzer β2M kinetics with cause-specific mortality in the HEMO cohort, focusing on cardiac and infectious deaths.
Concise Methods
HEMO Study Design
The HEMO Study was a randomized, multicenter clinical trial with a 2 × 2 factorial design and equal allocation to each treatment arm (1). A total of 1846 patients were randomly assigned to either a standard dosage of dialysis targeting an equilibrated Kt/V of 1.05 or a higher dosage targeting an equilibrated Kt/V of urea of 1.45 and to either low-flux or high-flux membrane dialyzers.
Among the eligibility criteria for entry into the study were (1) a minimum of 3 mo on hemodialysis and (2) a residual kidney urea clearance <1.5 ml/min per 35 L urea distribution volume. This latter criterion was included to minimize the contribution of clearance by native kidneys and hence maximize the relative effects of dialysis on total body solute clearance. Details of the study protocol have been described in previous reports (8,9). For this report, 1813 of the 1846 patients were included in the analyses of follow-up serum β2M levels; the remaining 33 patients were excluded because they did not have serum β2M measurements after randomization.
Dialyzers and Dialysates
The dialyzers used, frequency of serum sample collection for β2M, and calculation of clearances and Kt/V of β2M in the HEMO Study were previously reported (2,5,10). The criteria for high-flux dialyzers were an ultrafiltration coefficient ≥14 ml/h per mmHg and a β2M clearance >20 ml/min averaged over the lifespan of the dialyzer during clinical dialysis. The F80 (Fresenius Medical Care-North America, Lexington, MA) and CT190 (Baxter Healthcare Corp., McGaw Park, IL) dialyzers were used in 43 and 48% of the high-flux sessions, respectively. Low-flux dialyzers had a mean clearance of β2M <10 ml/min. The F8 (Fresenius) and CA210 (Baxter) dialyzers were used in 46 and 43% of the low-flux sessions, respectively.
All dialysate solutions were bicarbonate based. Standards for the quality of the dialysate water and the dialysates were those proposed by the Association for the Advancement of Medical Instrumentation. Ultrapure dialysate was not used.
Sample Collection for β2M Levels and Dialyzer β2M Kinetics
The β2M kinetics during hemodialysis were determined at the first and second months and then every other month during the follow-up period for patients in the high-flux arm. For the low-flux arm, β2M kinetics were determined at the first and fourth months and annually thereafter. Blood samples for β2M were collected immediately before dialysis and 20 s after dialysis from the dialyzer afferent blood tubing after the dialyzer blood flow rate had been reduced to <80 ml/min. Blood samples were centrifuged, and the serum samples were sent to a central laboratory (Spectra East, Rockleigh, NJ) for assay.
The concentrations of β2M were determined using a solid-phase competitive RIA with reagents supplied by Abbott Laboratories (Abbott Park, IL), and radioactivity was determined by a Micromedic Apex Automatic Counter (model 10600; ICN Biomedicals, Costa Mesa, CA). The intra-assay and interassay coefficients of variation were 3.6 and 5.0%, respectively.
Dialyzer clearance of β2M was calculated on the basis of the change in serum β2M concentration during the dialysis session as described previously (2,5,10,11). The Kt/V for β2M was calculated by multiplying the dialyzer β2M clearance by the treatment time and dividing the product by the postdialysis extracellular fluid volume, which was calculated as one third of the urea distribution volume estimated by urea kinetics. The β2M clearances associated with the majority of these dialyzers and specific reprocessing techniques have been described in detail in a previous publication from the HEMO Study (10). A variety of reprocessing techniques were used in the participating clinical centers, resulting in highly variable β2M clearances in the high-flux arm.
Follow-up and Outcomes
The planned mean duration of follow-up for mortality in this cohort of 1813 patients was 4.5 yr (range 0.9 to 6.6 yr); however, the actual mean follow-up was only 2.6 yr because of deaths, kidney transplantation, and transfers to dialysis units that did not participate in the HEMO Study. The protocols used for the collection and validation of outcome data have been previously described in detail (12–14). The primary outcome of the HEMO Study was all-cause mortality. The primary cause of death for each case was adjudicated by a central outcome committee. For this report, only deaths primarily as a result of cardiac and infectious causes were analyzed. As discussed, the rationale for selecting cardiac deaths was the apparent effect of high-flux dialysis on this outcome in the HEMO Study (1,2,12). Infectious deaths were selected because of the substantial literature implicating middle molecules in the pathogenesis of immunodeficiency in the dialysis patients (6,7,15–19). Furthermore, cardiac diseases and infections are the two most common causes of death in hemodialysis patients.
Statistical Analyses
To avoid confounding from various reuse limits of different dialyzer/reprocessing method combinations, dialyzer β2M clearance and β2M Kt/V for different dialyzer/reprocessing method combinations were based on averages of predicted values at each follow-up kinetic modeling session (5). The predicted dialyzer β2M clearance and β2M Kt/V were obtained by multiple regression analysis of the observed values on the type of dialyzer, reuse number, and type of reprocessing method, based on sessions in which the serum β2M levels were measured.
The association between the risk for infectious or cardiac mortality and serum β2M levels was investigated using a time-dependent Cox regression model (20), in which the HR for infectious or cardiac mortality at a given time point were related to the cumulative mean of the predialysis serum β2M levels throughout the follow-up period before that time point. In this report, follow-up time was censored at deaths from causes other than the specific cause being analyzed, transplantation, and 4 mo after the patient had been transferred to a dialysis unit that did not participate in the HEMO Study. Similar time-dependent Cox models were performed to relate cause-specific mortality with the cumulative mean of predicted dialyzer β2M Kt/V over time during follow-up. For these Cox regression models, all randomly assigned patients who had undergone any kinetic modeling sessions during follow-up (n = 1826) were included. The following basic set of 11 baseline factors were included as covariates in these analyses: The seven baseline factors prespecified in the HEMO Study protocol (age, gender, race, diabetes, years on dialysis, comorbidity index (index of coexistent disease [ICED]) [21], and serum albumin level), baseline kinetically modeled urea distribution volume, residual kidney urea clearance, dialyzer flux assignment, and ultrafiltration volume normalized by body weight. The cohort was further divided into two subgroups on the basis of the mean prestudy years on dialysis (3.7 yr), and similar Cox regression analyses were performed relating serum β2M levels or dialyzer β2M kinetics to cause-specific mortality, using the same covariates as in the corresponding analysis of the entire cohort.
Three sets of sensitivity analyses were performed to evaluate the robustness of the results by altering different aspects of the statistical models. First, all analyses were repeated after separating the cohort into subgroups that were assigned to the individual flux interventions to determine whether the results differed between patients who were assigned to the low-flux and high-flux arms. Second, the time-dependent Cox regressions relating infectious mortality or cardiac mortality to the cumulative mean serum β2M levels or to the cumulative mean predicted dialyzer β2M Kt/V were repeated after controlling for the same 11 covariates listed for the main analyses but with the most recently recorded follow-up serum albumin level, ICED, and residual kidney urea clearance used instead of the baseline measures of these three variables. Third, the Cox regression analyses relating infectious mortality or cardiac mortality to serum β2M were repeated using the cumulative mean predialysis serum β2M levels during only the first 4 mo of follow-up as the exposure variable instead of the cumulative mean throughout the entire follow-up period. This sensitivity analysis was restricted to patients who survived and remained in the trial for at least 4 mo and was performed also adjusting for the 11 baseline covariates listed for the main analyses. This model has the advantage that the exposure is evaluated during a constant time period at the start of the follow-up, albeit of a limited duration.
Results
Patient Characteristics
The baseline characteristics of the 1846 randomly assigned patients in the HEMO Study have been previously published (1,2,12). Our cohort was somewhat different from the cohort reported in a previous publication relating serum β2M levels to all-cause mortality (5). The previous cohort was composed of 1704 individuals who had β2M kinetic modeling performed at 1 mo of follow-up. Our cohort was composed of 1813 patients who had at least one follow-up serum β2M measurement; 56.0% were female, 62.9% were black, 44.5% had diabetes, and the mean ± SD age was 57.6 ± 14.1 yr (Table 1). The mean postdialysis weight was 69.2 ± 14.7 kg, and 59.9% of the patients were treated with high-flux dialyzers before entry to the study. Only 33.0% of the cohort had measurable residual urine output.
Table 1.
Baseline characteristics of cohort (n = 1813)a
| Characteristic | Value |
|---|---|
| Age (yr; mean ± SD) | 57.6 ± 14.1 |
| Female (%) | 56.0 |
| Black (%) | 62.9 |
| Diabetes (%) | 44.5 |
| Years on dialysis (mean ± SD) | 3.8 ± 4.4 |
| Postdialysis weight (kg; mean ± SD) | 69.2 ± 14.7 |
| Urea distribution volume (L; mean ± SD)b | 31.1 ± 6.0 |
| Comorbidity (ICED) score (mean ± SD)c | 2.0 ± 0.8 |
| Cardiac disease (%) | 79.9 |
| Serum albumin (g/dl; mean ± SD) | 3.6 ± 0.4 |
| Residual kidney urea clearance >0 (%) | 33.0 |
| Residual kidney urea clearance >0.75 ml/min/35 L (%) | 14.0 |
| High-flux dialysis (%) | 59.9 |
| Ultrafiltration volume/postdialysis weight (L/70 kg; mean ± SD) | 3.0 ± 1.1 |
| Hematocrit (%; mean ± SD) | 33.6 ± 4.5 |
| Venous catheters (%) | 5.6 |
ICED, index of coexisting disease.
Urea distribution volume as determined by kinetic modeling.
Severity score computed with diabetes excluded (21).
The cumulative mean predialysis serum β2M levels and cumulative mean β2M Kt/V during the entire follow-up period for the entire cohort and various subgroups are presented in Table 2. Among this entire cohort of 1813 patients, there were 789 all-cause deaths during follow-up, 315 of which were cardiac deaths and 180 of which were infectious deaths.
Table 2.
Cumulative mean predialysis serum β2M level and dialysis β2M kinetics
| Parameter | Cumulative Mean Predialysis Serum β 2M Levela
|
Cumulative Mean Predicted β 2M Kt/V a,b
|
||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| Entire cohort | 1813 | 37.6 | 11.9 | 1826 | 0.37 | 0.34 |
| High-flux arm | 905 | 33.6 | 9.2 | 910 | 0.66 | 0.21 |
| Low-flux arm | 908 | 41.5 | 12.9 | 916 | 0.07 | 0.07 |
| Prestudy dialysis duration >3.7 yr | 569 | 41.8 | 11.7 | 572 | 0.37 | 0.33 |
| Prestudy dialysis duration ≤3.7 yr | 1244 | 35.6 | 11.4 | 1254 | 0.36 | 0.34 |
Cumulative values throughout the entire follow-up period including the last available value before death.
Obtained by multiple regression analysis of the observed values on the type of dialyzer, reuse number, and type of reprocessing method
Predictors of Cardiac Mortality
A limited number of independent covariates were included in these Cox models for the primary purpose of examining the association between β2M level and cardiac or infectious deaths. All data presented and those shown in Tables 3 through 6 and Figures 1 and 2 are results of regression analysis that included all of these covariates. The presence of diabetes and increased age, number of years on dialysis, ICED score, and ultrafiltration volume (reflecting interdialytic weight gain) at baseline were positively associated with cardiac mortality, whereas black race and increased baseline serum albumin concentration were inversely associated (Table 3).
Table 3.
Cox regression analysis of cardiac mortalitya
| Baseline Variable | HR | 95% CI | P |
|---|---|---|---|
| Cumulative mean predialysis serum β 2M level during follow-up (per 10-mg/L increase) | 1.10 | 0.99 to 1.21 | 0.079 |
| Age (per 10-yr increase) | 1.51 | 1.35 to 1.68 | <0.001 |
| Gender (female) | 0.83 | 0.63 to 1.10 | 0.20 |
| Race (black) | 0.61 | 0.46 to 0.80 | <0.001 |
| Diabetes | 1.88 | 1.45 to 2.44 | <0.001 |
| Years on dialysis (per 1-yr increase) | 1.04 | 1.01 to 1.07 | 0.011 |
| Urea distribution volume (per 1-L increase)b | 1.01 | 0.98 to 1.03 | 0.43 |
| ICED score (per 1-U increase)c | 1.29 | 1.12 to 1.50 | 0.001 |
| Serum albumin (per 1-g/dl increase) | 0.49 | 0.35 to 0.70 | <0.001 |
| Residual kidney urea clearance (per 1-ml/min increase)d | 0.83 | 0.61 to 1.12 | 0.23 |
| High-flux dialysis | 1.17 | 0.80 to 1.71 | 0.42 |
| Ultrafiltration volume/postdialysis weight (per 1-L/70-kg increase) | 1.19 | 1.07 to 1.33 | 0.001 |
Analysis stratified by clinical center; n = 1813. The HR for the cumulative mean predialysis serum β2M level remained 1.10 per 10-mg/L increase, after adjustment for baseline use of venous catheters and baseline hematocrit in addition to the covariates included the table. CI, confidence interval; HR, hazard ratio.
Urea distribution volume as determined by kinetic modeling.
Severity score computed with diabetes excluded (21).
Normalized to 35 L of urea distribution volume.
Table 4.
Cox regression analysis of infectious mortalitya
| Baseline Variable | HR | 95% CI | P |
|---|---|---|---|
| Cumulative mean predialysis serum β 2M level during follow-up (per 10-mg/L increase) | 1.21 | 1.07 to 1.37 | 0.002 |
| Age (per 10-yr increase) | 1.56 | 1.35 to 1.81 | <0.001 |
| Gender (female) | 1.12 | 0.77 to 1.63 | 0.54 |
| Race (black) | 0.79 | 0.55 to 1.15 | 0.22 |
| Diabetes | 1.34 | 0.95 to 1.88 | 0.09 |
| Years on dialysis (per 1-yr increase) | 1.04 | 1.00 to 1.08 | 0.045 |
| Urea distribution volume (per 1-L increase)b | 0.99 | 0.96 to 1.03 | 0.70 |
| ICED score (per 1-U increase)c | 1.41 | 1.16 to 1.72 | 0.001 |
| Serum albumin (per 1-g/dl increase) | 0.50 | 0.31 to 0.80 | 0.004 |
| Residual kidney urea clearance (per 1-ml/min increase)d | 1.11 | 0.77 to 1.62 | 0.57 |
| High-flux dialysis | 0.74 | 0.46 to 1.21 | 0.24 |
| Ultrafiltration volume/postdialysis weight (per 1-L/70-kg increase) | 1.25 | 1.08 to 1.45 | 0.002 |
Adjusted for clinical center; n = 1813. The HR for the cumulative mean predialysis serum β2M level remained 1.21 per 10-mg/L increase, after adjustment for baseline use of venous catheters and baseline hematocrit in addition to the covariates included the table.
Urea distribution volume as determined by kinetic modeling.
Severity score computed with diabetes excluded (21).
Normalized to 35 L of urea distribution volume.
Table 5.
Association of cumulative mean predialysis serum β2M levels during follow-up with cardiac mortality or infectious mortalitya
| Parameter | n | Cardiac Mortality
|
Infectious Mortality
|
||||
|---|---|---|---|---|---|---|---|
| HR (per 10-mg/L Increase) | 95% CI | P | HR (per 10-mg/L Increase) | 95% CI | P | ||
| Entire cohort | 1813 | 1.10 | 0.99 to 1.21 | 0.079 | 1.21 | 1.07 to 1.37 | 0.002 |
| Subgroup with ≤3.7 yr of dialysis | 1244 | 1.08 | 0.93 to 1.24 | 0.31 | 1.22 | 1.03 to 1.45 | 0.023 |
| Subgroup with >3.7 yr of dialysis | 569 | 1.10 | 0.93 to 1.30 | 0.26 | 1.24 | 1.00 to 1.54 | 0.050 |
Adjusted for baseline age, gender, race, diabetes, years on dialysis, kinetically modeled urea distribution volume, comorbidity index, serum albumin level, residual kidney urea clearance, dialyzer flux, and ultrafiltration volume normalized by body weight.
Table 6.
Association of cumulative mean β2M Kt/V during follow-up with cardiac mortality or infectious mortalitya
| Parameter | n | Cardiac Mortality
|
Infectious Mortality
|
||||
|---|---|---|---|---|---|---|---|
| HR (per 0.1-U Increase) | 95% CI | P | HR (per 0.1-U Increase) | 95% CI | P | ||
| Entire cohort | 1826 | 0.97 | 0.94 to 1.01 | 0.11 | 0.98 | 0.93 to 1.02 | 0.34 |
| Subgroup with ≤3.7 yr of dialysis | 1254 | 1.00 | 0.95 to 1.04 | 0.82 | 0.99 | 0.94 to 1.05 | 0.77 |
| Subgroup with >3.7 yr of dialysis | 572 | 0.93 | 0.87 to 1.00 | 0.036 | 0.93 | 0.86 to 1.01 | 0.10 |
Adjusted for baseline age, gender, race, diabetes, years on dialysis, kinetically modeled urea distribution volume, comorbidity index, serum albumin level, residual kidney urea clearance, dialyzer flux, and ultrafiltration volume normalized by body weight.
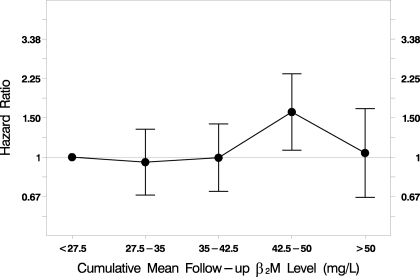
Figure 1.
Association of predialysis serum β2-microglobulin (β2M) levels with cardiac mortality. Cumulative mean predialysis serum β2M levels during the entire follow-up period were not significantly associated with cardiac mortality (hazard ratio [HR] 1.10 per 10-mg/L increase; P = 0.079; n = 1813). The statistical analysis was performed using a time-dependent Cox regression model, adjusted for baseline age, gender, race, diabetes, comorbidity index, kinetically modeled urea distribution volume, prestudy duration of dialysis, serum albumin level, residual kidney urea clearance, dialyzer flux, and ultrafiltration volume normalized by body weight.
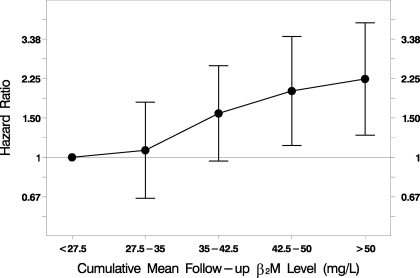
Figure 2.
Association of predialysis serum β2M levels with infectious mortality. Cumulative mean predialysis serum β2M levels during the entire follow-up period were significantly associated with infectious mortality (HR 1.21 per 10-mg/L increase; P = 0.002; n = 1813). The statistical analysis was performed using a time-dependent Cox regression model, adjusted for baseline age, gender, race, diabetes, comorbidity index, kinetically modeled urea distribution volume, prestudy duration of dialysis, serum albumin level, residual kidney urea clearance, dialyzer flux, and ultrafiltration volume normalized by body weight.
Predictors of Infectious Mortality
The factors that were associated with infectious mortality were similar to those that were associated with cardiac mortality. Increased age, number of years on dialysis, ICED score, and ultrafiltration volume at baseline were positively associated with infectious death, whereas baseline serum albumin level was inversely associated (Table 4). The presence of diabetes was also associated with infectious deaths, and black race was inversely associated with infectious deaths, but these associations were not statistically significant (Table 4), in contrast to cardiac death (Table 3).
Predictive Values of Serum β2M Levels for Cardiac Mortality
There was no statistically significant association between cumulative mean predialysis serum β2M level and cardiac mortality in the entire cohort (Tables 3 and 5, Figure 1) or the two dialysis duration subgroups (Table 5).
Predictive Values of Serum β2M Levels for Infectious Mortality
In the entire cohort, each 10-mg/L increase in serum β2M level was associated with a 21% increase in the rate of infectious mortality (HR 1.21; 95% CI 1.07 to 1.37; P = 0.002; Tables 4 and 5, Figure 2). Associations of similar magnitude, albeit with lower statistical significance, were also observed in the two dialysis duration subgroups (Table 5).
Predictive Values of Dialyzer β2M Kt/V for Cardiac Mortality
Similar Cox regression models were used to relate dialyzer β2M Kt/V with cause-specific mortality. These models also included the predictor variables presented in Tables 3 and 4, except for serum β2M levels. Results of these analyses of dialyzer β2M Kt/V (Table 6) were different from those that were obtained from analysis of serum β2M levels. The Kt/V of β2M correlated significantly with cardiac mortality in the subgroup of patients who had been on dialysis for >3.7 yr before the study. In this subgroup, each 0.1-U increase in β2M Kt/V was associated with a 7% decrease in the rate of cardiac mortality (HR 0.93; 95% CI 0.87 to 1.00; P = 0.036). There were no statistically significant associations between β2M Kt/V and cardiac mortality in the entire cohort or those who had been on dialysis for ≤3.7 yr before the study.
Predictive Values of Dialyzer β2M Kt/V for Infectious Mortality
Results of the analyses of dialyzer β2M Kt/V with infectious mortality (Table 6) also exhibited a different pattern from the results of the analysis of serum β2M levels. The Kt/V of β2M was not significantly associated with infectious mortality in either the full cohort or the two dialysis duration subgroups. It should be noted, however, that the HR of 0.93 for infectious mortality in the long-term dialysis subgroup was similar to the HR relating cardiac mortality to dialyzer β2M Kt/V but did not reach statistical significance because the number of infectious deaths was smaller than the number of cardiac deaths, leading to a lower statistical power for the infectious death outcome.
Sensitivity Analyses
Three sets of sensitivity analyses were performed to evaluate the robustness of the results. In the first set of sensitivity analyses, in which the cohort was examined separately by flux assignments, there were no significant differences between the low-flux arm and high-flux arms in the adjusted HR relating cardiac or infectious mortality to either the cumulative mean serum β2M level or the cumulative mean β2M Kt/V. The HR relating infectious and cardiac mortality to cumulative mean serum β2M were 1.36 (95% CI 1.05 to 1.76) and 1.12 (95% CI 0.90 to 1.38) per 10-g/dl increase, respectively, in the high-flux arm and were 1.17 (95% CI 0.99 to 1.39) and 1.06 (95% CI 0.93 to 1.21) per 10-g/dl increase, respectively, in the low-flux arm.
In the second set of sensitivity analyses, the most recently recorded follow-up serum albumin level, ICED, and residual kidney urea clearance were used for adjustment in the Cox regression models, instead of the baseline values of these variables. In these analyses, the adjusted HR of relating infectious mortality to cumulative mean serum β2M in the full cohort was 1.14 (95% CI 1.01 to 1.29; P = 0.039), which remained statistically significant. The HR relating cardiac death to cumulative mean serum β2M and the HR relating both infections and cardiac death to cumulative mean β2M Kt/V were similar when adjusting for the follow-up or baseline values of these variables (data not shown).
In a third set of sensitivity analyses, the mean serum β2M level and β2M Kt/V during the first 4 mo of follow-up, instead of the entire follow-up period, were used as the exposure variables. In these analyses, the adjusted HR relating cardiac and infectious mortality to serum β2M were 1.05 (95% CI 0.95 to 1.15) and 1.14 (95% CI 1.01 to 1.28) per 10-mg/L increase, respectively, and the adjusted HR relating cardiac and infectious mortality to β2M Kt/V were 0.97 (95% CI 0.94 to 1.00) and 0.93 (95% CI 0.97 to 1.02) per 0.10-Kt/V-unit increase, respectively.
Discussion
The results of this study show that the cumulative mean predialysis serum level of the middle molecule, β2M, correlated positively with the relative risk for infectious deaths in the HEMO Study. Middle molecules have been generally defined as solutes that accumulate as a result of kidney failure and do not follow the kinetics of clearance by conventional low-flux hemodialysis membranes. To qualify as toxic, a middle molecule would have an additional requirement of demonstrable tissue toxicity in vitro and preferably systemic toxicity in vivo. An even more stringent criterion would be demonstrable adverse impacts on clinical outcomes. Numerous middle molecules that satisfy these various criteria have been identified, categorized, and reviewed (22,23).
Of particular relevance to this analysis are the group of middle molecules that have granulocyte-inhibitory properties in vitro and include granulocyte-inhibitory protein II (MW approximately 9500 Da with homology to β2M) (6), chemotaxis inhibitory protein (MW approximately 8500 Da with homology to ubiquitin) (19), degranulation inhibitory protein (MW approximately 14,000 Da with homology to angiogenin) (24), and granulocyte inhibitory protein I (MW approximately 28,000 Da with homology to the light chain of IgG) (16). By inhibiting granulocyte function, these serum proteins could predispose patients with kidney failure to infectious complications. In support of the pathogenic importance of these proteins, these analyses show that the serum level of β2M, a protein with a similar MW of 11,800 Da, was associated with deaths from infectious causes (Tables 4 and 5).
In these analyses, the mean of the predialysis serum β2M levels throughout follow-up was related to the relative risks for mortality of specific causes. This averaged β2M value presumably reflected the cumulative effects of other middle molecules for which β2M serves as a surrogate. It should be emphasized that this predictive value of β2M for infectious mortality was independent of residual kidney function (Table 4), further supporting the importance of this middle molecule. The effect size was substantial; for a 10-mg/L increase in the serum β2M level, there was a 21% increase in the relative risk for infectious death, compared with the 11% increase in the relative risk for all-cause mortality previously reported by in the HEMO Study (5). Moreover, this predictive value of infectious mortality and effect size were independent of the duration of previous dialysis and was consistent in the entire cohort, in the subgroup with ≤3.7 yr of previous dialysis, and in the long-term dialysis subgroup (Table 5).
In contrast to infectious deaths, serum β2M levels were not associated with increased risk for cardiac death in the entire cohort or in the two subgroups defined by prestudy dialysis durations (Tables 3 and 5). In addition, the relationships between dialytic β2M removal and clinical outcomes were different. Previous secondary analysis of the HEMO Study data showed that the reduction in the relative risk for infectious death associated with randomization to high-flux dialysis was not statistically significant in the entire cohort (15%) and in the long-term (>3.7 yr) dialysis subgroup (22%) (13). In contrast, randomization to the high-flux arm was associated with a lower risk for cardiac death in the entire cohort (1,2). Furthermore, in the subgroup that had undergone dialysis for >3.7 yr, there was a statistically significant decrease in all-cause mortality by 32%, cardiac mortality by 37%, and cerebrovascular events by 71% (3) in the high-flux arm. If high-flux dialysis is in fact associated with better clinical outcomes, then it is likely to be mediated by the reduction of middle molecules in the uremic serum, because this is a major difference between high flux and low flux that is in favor of the former (5). Indeed, high-flux dialysis removes middle molecules (25,26) that potentially contribute to cardiovascular diseases, for example, advanced glycation end products that are putatively atherogenic (27). Differences in biocompatibility may, in some instances, potentially account for the differences in clinical outcomes among various dialysis membranes. The membrane materials used in the HEMO Study, however, were largely similar in biocompatibility between the high-flux and low-flux arms (2,10). In addition, back-filtration with the accompanying transfer of dialysate contaminants into the blood stream is more likely in high flux; however, such a phenomenon would be expected to worsen instead of improve the outcomes
Because β2M was used as the marker to define membrane flux in the HEMO Study, it seems appropriate to examine the effect of β2M removal on clinical outcomes. We selected β2M Kt/V instead of dialyzer β2M clearance in these analyses, because the latter does not account for the dialysis time and therefore the total β2M removed during the session. Our recent analysis showed that β2M Kt/V was inversely associated with all-cause mortality only in the subgroup of patients who had been on dialysis for >3.7 yr but not in the entire cohort or the short-term dialysis subgroup (5). Consistent with this observation, this analysis shows that β2M Kt/V was also inversely associated with cardiac death and infectious death in the long-term dialysis subgroup, although the association was not statistically significant for the infectious death outcome (Table 6). It should be noted that these are observational analyses of β2M removal and not the conventional intention-to-treat analysis for randomized trials. Nonetheless, there seemed to be discrepancies between the effects of β2M removal on clinical outcomes and the effects of serum β2M levels on clinical outcomes, raising the possibility that generation, in addition to the removal, of β2M or other similar middle molecules also contributes to the clinical outcomes.
There are limitations to this analysis. First, whereas most of the data in the HEMO Study were collected prospectively as part of the randomized trial, the secondary analysis correlating serum β2M levels or dialyzer β2M Kt/V with clinical outcomes in this report was a post hoc plan. Second, our database is derived from a clinical trial that had exclusion criteria for enrollment; therefore, generalization of these results to the US hemodialysis population should take this caveat into account. The third limitation relates to the lack of definitive evidence to ascertain cardiac causes of death in cases of unwitnessed sudden death.
Conclusions
Although there were some apparent inconsistencies among the various analyses relating β2M to clinical outcomes, the data generally support the role of β2M as a marker of uremic middle molecules. In addition, the association between serum β2M levels and infectious death further validates the importance of serum middle-MW proteins in the pathogenesis of immunodeficiency in advanced kidney failure. This hypothesis and the utility of β2M as a guide for maintenance dialysis adequacy should be further explored.
Disclosures
None.
Acknowledgments
The original trial (HEMO Study) and this retrospective analysis were supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.
We are indebted to the patients and study coordinators in all clinical centers who participated in the HEMO Study.
Published online ahead of print. Publication date available at www.cjasn.org.
Dr. John K. Leypoldt is currently an employee of Baxter Healthcare Corporation (McGraw Park, Illinois).
References
- 1.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, Dwyer JT, Levey AS, Levin NW, Milford E, Ornt DB, Rocco MV, Schulman G, Schwab SJ, Teehan BP, Toto R: Hemodialysis (HEMO) Study Group: Effect of dialysis dose and membrane flux on mortality and morbidity in maintenance hemodialysis patients—Primary results of the HEMO study. N Engl J Med 347: 2010–2019, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Cheung AK, Levin NW, Greene T, Agodoa L, Bailey J, Beck G, Clark W, Levey AS, Leypoldt JK, Ornt DB, Rocco MV, Schulman G, Schwab S, Teehan B, Eknoyan G, HEMO Study Group: Effect of high-flux hemodialysis on clinical outcomes: Results of the HEMO Study. J Am Soc Nephrol 14: 3251–3263, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Delmez JA, Yan G, Bailey J, Beck GJ, Beddhu S, Cheung AK, Kaysen GA, Levey AS, Sarnak MJ, Schwab SJ: Hemodialysis (HEMO) Study Group: Cerebrovascular disease in maintenance hemodialysis patients—Results of the HEMO Study. Am J Kidney Dis 47: 131–138, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Greene T: What did we learn from the HEMO Study? Implications of secondary analyses. Contrib Nephrol 149: 1–14, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Cheung AK, Rocco MV, Yan G, Leypoldt JK, Levin NW, Greene T, Agodoa L, Bailey J, Beck GJ, Clark W, Levey AS, Ornt DB, Schulman G, Schwab S, Teehan B, Eknoyan G, HEMO Study Group: Serum beta-2-microglobulin levels predict mortality in dialysis patients: Results of the HEMO study. J Am Soc Nephrol 17: 546–555, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Haag-Weber M, Mai B, Hörl WH: Isolation of a granulocyte inhibitory protein from uraemic patients with homology of beta 2-microglobulin. Nephrol Dial Transplant 9: 382–388, 1994 [PubMed] [Google Scholar]
- 7.Haag-Weber M, Mai B, Hörl WH: Impaired cellular host defense in peritoneal dialysis by two granulocyte inhibitory proteins. Nephrol Dial Transplant 9: 1769–1773, 1994 [PubMed] [Google Scholar]
- 8.Eknoyan G, Levey AS, Beck GJ, Agodoa LY, Daugirdas JT, Kusek JW, Levin NW, Schulman G, the HEMO Study Group: The Hemodialysis (HEMO) Study: Rationale for selection of interventions. Semin Dial 9: 24–33, 1996 [Google Scholar]
- 9.Greene T, Beck GJ, Gassman JJ, Gotch FA, Kusek JW, Levey AS, Levin NW, Schulman G, Eknoyan G: Design and statistical issues of the hemodialysis (HEMO) study. Control Clin Trials 21: 502–525, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Cheung AK, Agodoa LY, Daugirdas JT, Depner TA, Gotch FA, Greene T, Levin NW, Leypoldt JK, the Hemodialysis (HEMO) Study: Effects of hemodialyzer reuse on clearances of urea and β2-microglobulin. J Am Soc Nephrol 10: 117–127, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Leypoldt JK, Deeter RB, Cheung AK: Single compartment models for evaluating β2M clearance during hemodialysis. ASAIO J 43: 904–909, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Cheung AK, Sarnak M, Yan G, Berkoben M, Heyka R, Kaufman A, Lewis J, Rocco M, Toto R, Windus D, Ornt D, Levey AS, HEMO Study Group: Cardiac diseases in maintenance hemodialysis patients: Results of the HEMO Study. Kidney Int 65: 2380–2389, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Allon M, Depner TA, Radeva M, Bailey J, Beddhu S, Butterly D, Coyne DW, Gassman JJ, Kaufman AM, Kaysen GA, Lewis JA, Schwab SJ, HEMO Study Group: Impact of dialysis dose and membrane on infection-related hospitalization and death: Results of the HEMO Study. J Am Soc Nephrol 14: 1863–1870, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Rocco MV, Yan G, Gassman J, Lewis JB, Ornt D, Weiss B, Levey AS, Hemodialysis Study Group: Comparison of causes of death using HEMO Study and HCFA end-stage renal disease death notification classification systems. Am J Kidney Dis 39: 146–153, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Hörl WH, Haag-Weber M, Georgopoulos A, Block LH: Physicochemical characterization of a polypeptide present in uremic serum that inhibits the biological activity of polymorphonuclear cells. Proc Natl Acad Sci U S A 87: 6353–6357, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen G, Haag-Weber M, Mai B, Deicher R, Hörl WH: Effect of immunoglobulin light chains from hemodialysis and continuous ambulatory peritoneal dialysis patients on polymorphonuclear leukocyte functions. J Am Soc Nephrol 6: 1592–1599, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Haag-Weber M, Hörl WH: Are granulocyte inhibitory proteins contributing to enhanced susceptibility to infections in uraemia? Nephrol Dial Transplant Suppl 11: S98–S100, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Cohen G, Haag-Weber M, Hörl WH: Immune dysfunction in uremia. Kidney Int Suppl 62: S79–S82, 1997 [PubMed] [Google Scholar]
- 19.Cohen G, Rudnicki M, Hörl WH: Isolation of modified ubiquitin as a neutrophil chemotaxis inhibitor from uremic patients. J Am Soc Nephrol 9: 451–456, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Cox DR: Regression models and life tables (with discussion). J R Stat Soc Series B 34: 187–220, 1972 [Google Scholar]
- 21.Miskulin DC, Athienites NV, Yan G, Martin AA, Ornt DB, Kusek JW, Meyer KB, Levey AS, the Hemodialysis (HEMO) Study Group: Comorbidity assessment using the index of coexistent disease in a multicenter clinical trial Study. Kidney Int 60: 1498–1510, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Vanholder R, De Smet R, Glorieux G, Argiles A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jorres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W, European Uremic Toxin Work Group (EUTox): Review on uremic toxins: Classification, concentration, and interindividual variabil-ity. Kidney Int 63: 1934–1943, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Boure T, Vanholder R: Biochemical and clinical evidence for uremic toxicity. Artif Organs 28: 248–253, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Schmaldienst S, Oberpichler A, Tschesche H, Hörl WH: Angiogenin: A novel inhibitor of neutrophil lactoferrin release during extracorporeal circulation. Kidney Blood Press Res 26: 107–112, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Makita Z, Radoff S, Rayfield EJ, Yang Z, Skolnik E, Delaney V, Friedman EA, Cerami A, Vlassara H: Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med 325: 836–842, 1991 [DOI] [PubMed] [Google Scholar]
- 26.Weiss MF, Erhard P, Kader-Attia FA, Wu YC, Deoreo PB, Araki A, Glomb MA, Monnier VM: Mechanisms for the formation of glycoxidation products in end-stage renal disease. Kidney Int 57: 2571–2585, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Makita Z, Yanagisawa K, Kuwajima S, Bucala R, Vlassara H, Koike T: The role of advanced glycosylation end-products in the pathogenesis of atherosclerosis. Nephrol Dial Transplant 11[Suppl 5]: 31–33, 1996 [DOI] [PubMed] [Google Scholar]