Abstract
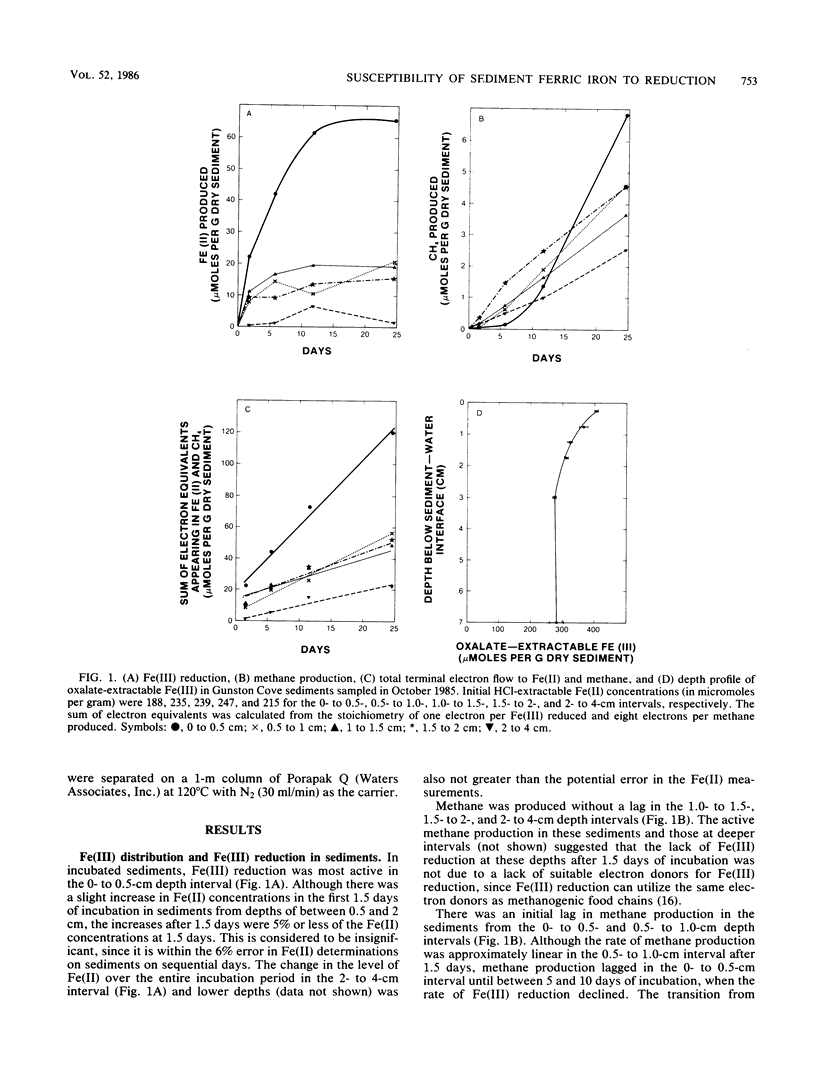
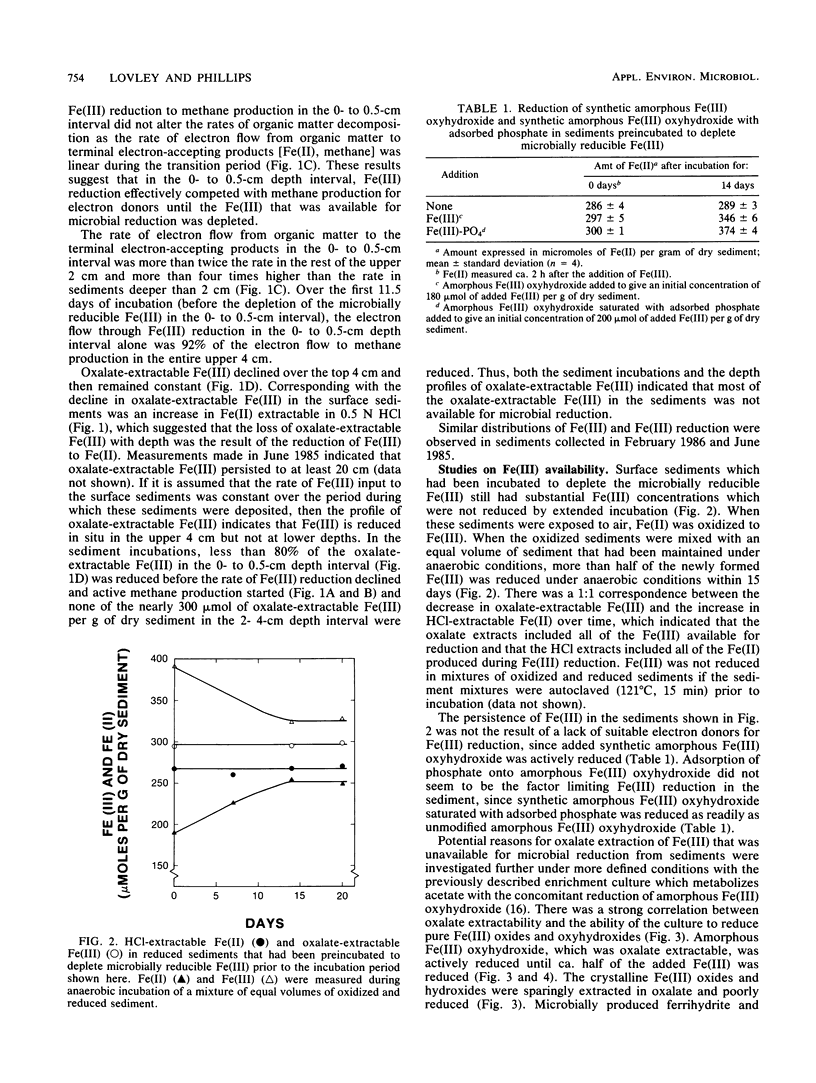
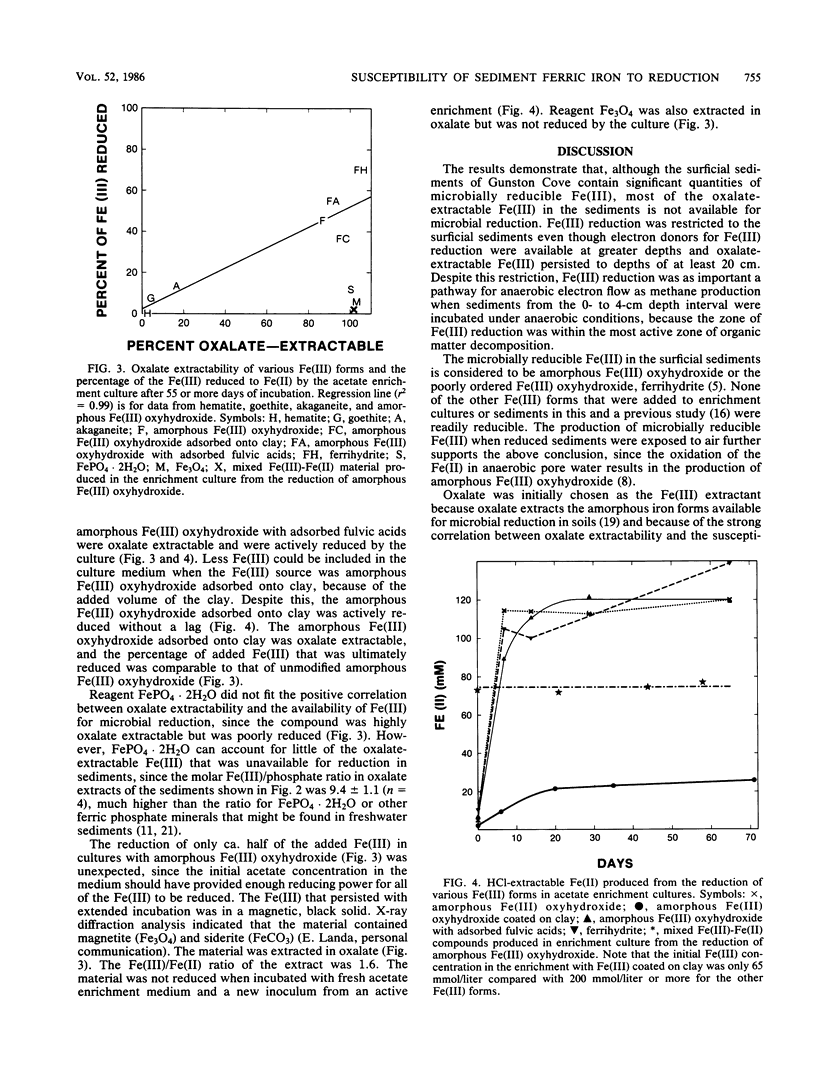
The distribution of Fe(III), its availability for microbial reduction, and factors controlling Fe(III) availability were investigated in sediments from a freshwater site in the Potomac River Estuary. Fe(III) reduction in sediments incubated under anaerobic conditions and depth profiles of oxalate-extractable Fe(III) indicated that Fe(III) reduction was limited to depths of 4 cm or less, with the most intense Fe(III) reduction in the top 1 cm. In incubations of the upper 4 cm of the sediments, Fe(III) reduction was as important as methane production as a pathway for anaerobic electron flow because of the high rates of Fe(III) reduction in the 0- to 0.5-cm interval. Most of the oxalate-extractable Fe(III) in the sediments was not reduced and persisted to a depth of at least 20 cm. The incomplete reduction was not the result of a lack of suitable electron donors. The oxalate-extractable Fe(III) that was preserved in the sediments was considered to be in a form other than amorphous Fe(III) oxyhydroxide, since synthetic amorphous Fe(III) oxyhydroxide, amorphous Fe(III) oxyhydroxide adsorbed onto clay, and amorphous Fe(III) oxyhydroxide saturated with adsorbed phosphate or fulvic acids were all readily reduced. Fe3O4 and the mixed Fe(III)-Fe(II) compound(s) that were produced during the reduction of amorphous Fe(III) oxyhydroxide in an enrichment culture were oxalate extractable but were not reduced, suggesting that mixed Fe(III)-Fe(II) compounds might account for the persistence of oxalate-extractable Fe(III) in the sediments. The availability of microbially reducible Fe(III) in surficial sediments demonstrates that microbial Fe(III) reduction can be important to organic matter decomposition and iron geochemistry. However, the overall extent of microbial Fe(III) reduction is governed by the inability of microorganisms to reduce most of the Fe(III) in the sediment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Lovley D. R., Phillips E. J. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol. 1986 Apr;51(4):683–689. doi: 10.1128/aem.51.4.683-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J. Reduction of ferric iron in anaerobic, marine sediment and interaction with reduction of nitrate and sulfate. Appl Environ Microbiol. 1982 Feb;43(2):319–324. doi: 10.1128/aem.43.2.319-324.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. C. Suboxic diagenesis in banded iron formations. Nature. 1984 May 24;309:340–342. doi: 10.1038/309340a0. [DOI] [PubMed] [Google Scholar]


