Abstract
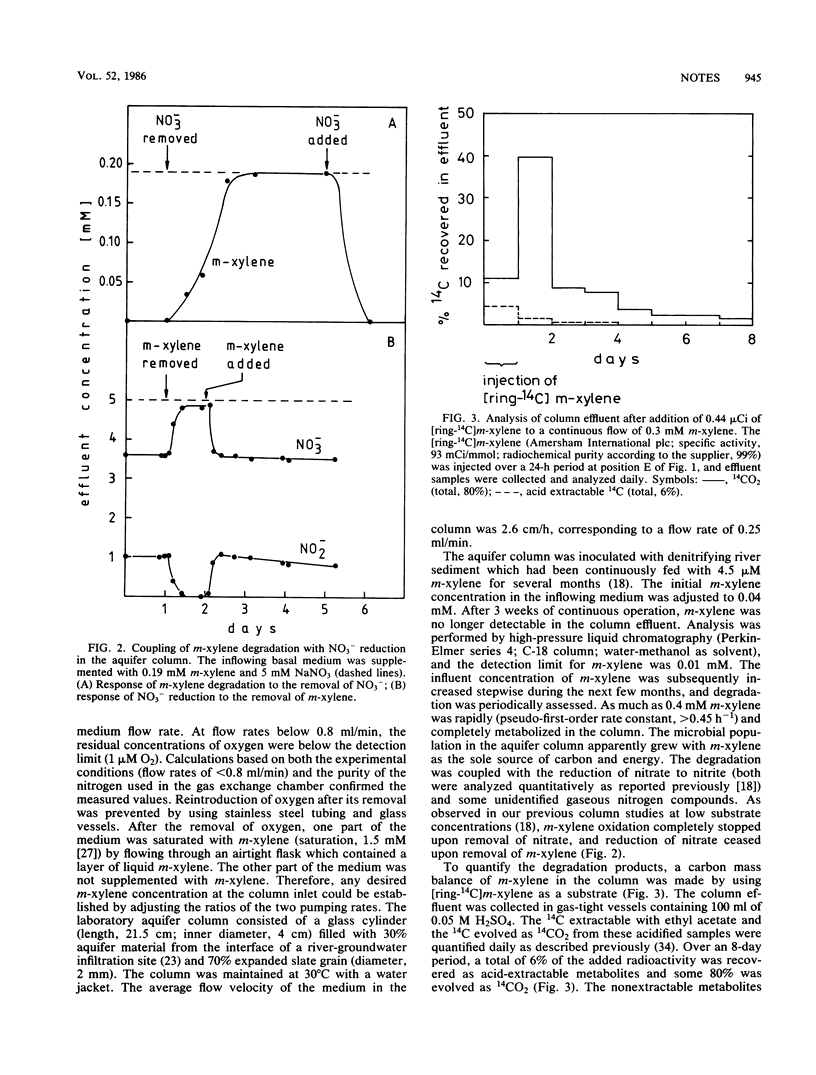
Up to 0.4 mM 1,3-dimethylbenzene (m-xylene) was rapidly mineralized in a laboratory aquifer column operated in the absence of molecular oxygen with nitrate as an electron acceptor. Under continuous flow conditions, the degradation rate constant (pseudo-first order) was >0.45 h−1. Based on a carbon mass balance with [ring-14C]m-xylene and a calculation of the electron balance, m-xylene was shown to be quantitatively (80%) oxidized to CO2 with a concomitant reduction of nitrate. The mineralization of m-xylene in the column also took place after reducing the redox potential, E′, of the inflowing medium with sulfide to <−0.11 V. Microorganisms adapted to growth on m-xylene were also able to degrade toluene under denitrifying conditions. These results suggest that aromatic hydrocarbons present in anoxic environments such as lake sediments, sludge digestors, and groundwater infiltration zones from landfills and polluted rivers are not necessarily persistent but may be mineralized in the absence of molecular oxygen.
Full text
PDF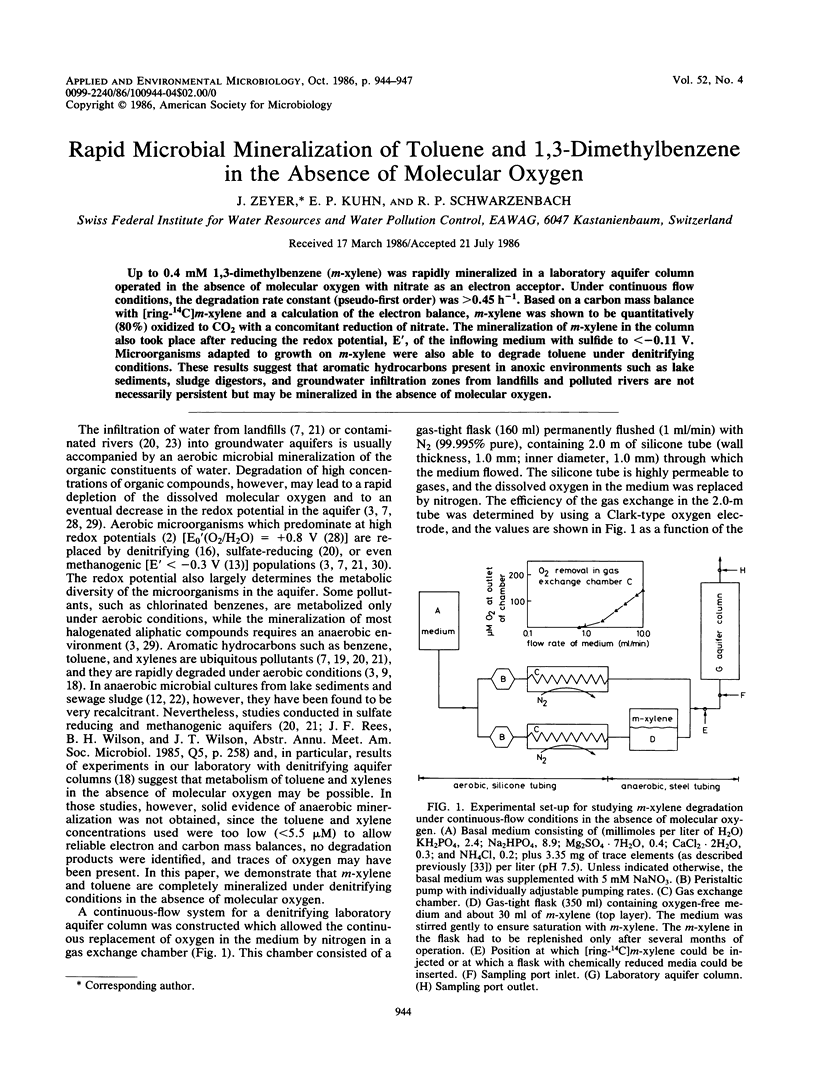


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alperin M. J., Reeburgh W. S. Inhibition experiments on anaerobic methane oxidation. Appl Environ Microbiol. 1985 Oct;50(4):940–945. doi: 10.1128/aem.50.4.940-945.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill D. L., Ghiorse W. C. Characterization of subsurface bacteria associated with two shallow aquifers in oklahoma. Appl Environ Microbiol. 1985 Sep;50(3):580–588. doi: 10.1128/aem.50.3.580-588.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd S. A., Shelton D. R. Anaerobic biodegradation of chlorophenols in fresh and acclimated sludge. Appl Environ Microbiol. 1984 Feb;47(2):272–277. doi: 10.1128/aem.47.2.272-277.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun K., Gibson D. T. Anaerobic degradation of 2-aminobenzoate (anthranilic acid) by denitrifying bacteria. Appl Environ Microbiol. 1984 Jul;48(1):102–107. doi: 10.1128/aem.48.1.102-107.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colberg P. J., Young L. Y. Aromatic and Volatile Acid Intermediates Observed during Anaerobic Metabolism of Lignin-Derived Oligomers. Appl Environ Microbiol. 1985 Feb;49(2):350–358. doi: 10.1128/aem.49.2.350-358.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C. Biochemistry of the bacterial catabolism of aromatic compounds in anaerobic environments. Nature. 1977 Nov 3;270(5632):17–22. doi: 10.1038/270017a0. [DOI] [PubMed] [Google Scholar]
- Grbić-Galić D. Fermentative and oxidative transformation of ferulate by a facultatively anaerobic bacterium isolated from sewage sludge. Appl Environ Microbiol. 1985 Oct;50(4):1052–1057. doi: 10.1128/aem.50.4.1052-1057.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper D. J. Incorporation of [18O]water in the formation of p-hydroxybenzyl alcohol by the p-cresol methylhydroxylase from Pseudomonas putida. Biochem J. 1978 Oct 1;175(1):345–347. doi: 10.1042/bj1750345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. Denitrification. Microbiol Rev. 1982 Mar;46(1):43–70. doi: 10.1128/mr.46.1.43-70.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton D. R., Tiedje J. M. Isolation and partial characterization of bacteria in an anaerobic consortium that mineralizes 3-chlorobenzoic Acid. Appl Environ Microbiol. 1984 Oct;48(4):840–848. doi: 10.1128/aem.48.4.840-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J., Tiedje J. M., Firestone R. B. Inhibition by sulfide of nitric and nitrous oxide reduction by denitrifying Pseudomonas fluorescens. Appl Environ Microbiol. 1980 Jan;39(1):105–108. doi: 10.1128/aem.39.1.105-108.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. F. Aerobic and Anaerobic Catabolism of Vanillic Acid and Some Other Methoxy-Aromatic Compounds by Pseudomonas sp. Strain PN-1. Appl Environ Microbiol. 1983 Dec;46(6):1286–1292. doi: 10.1128/aem.46.6.1286-1292.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnder A. J., Brock T. D. Methane formation and methane oxidation by methanogenic bacteria. J Bacteriol. 1979 Jan;137(1):420–432. doi: 10.1128/jb.137.1.420-432.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyer J., Wasserfallen A., Timmis K. N. Microbial mineralization of ring-substituted anilines through an ortho-cleavage pathway. Appl Environ Microbiol. 1985 Aug;50(2):447–453. doi: 10.1128/aem.50.2.447-453.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


