Abstract
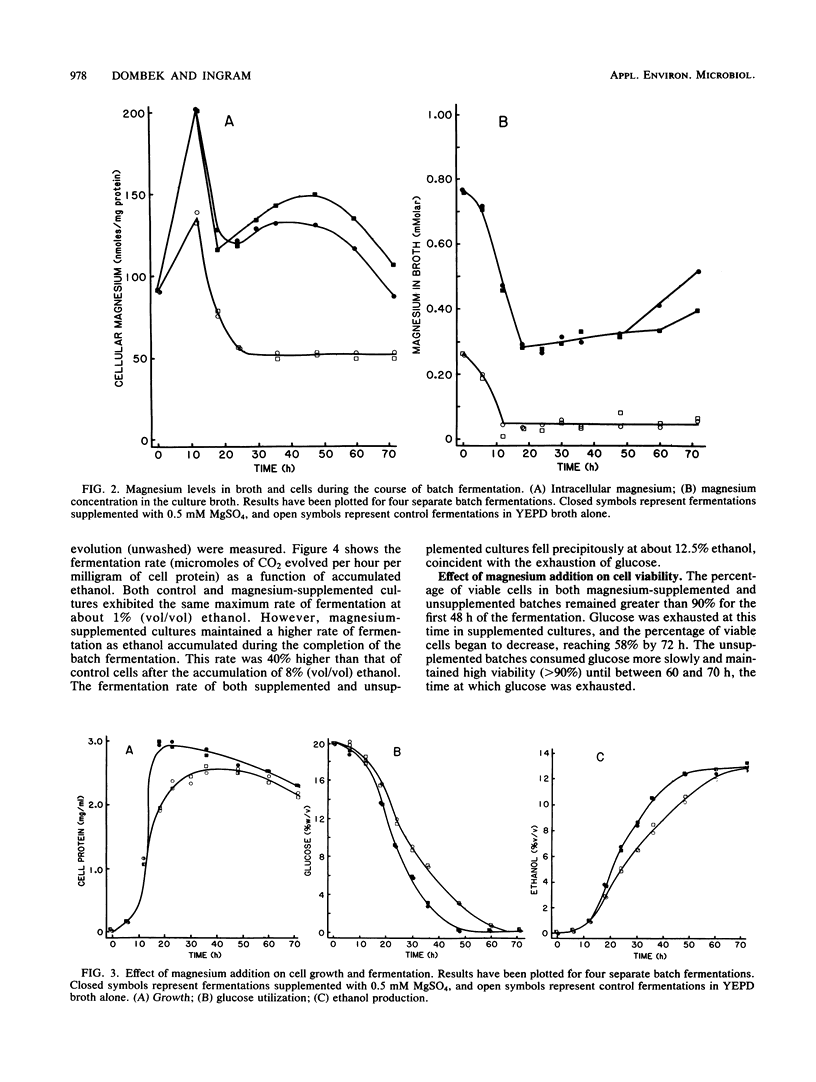
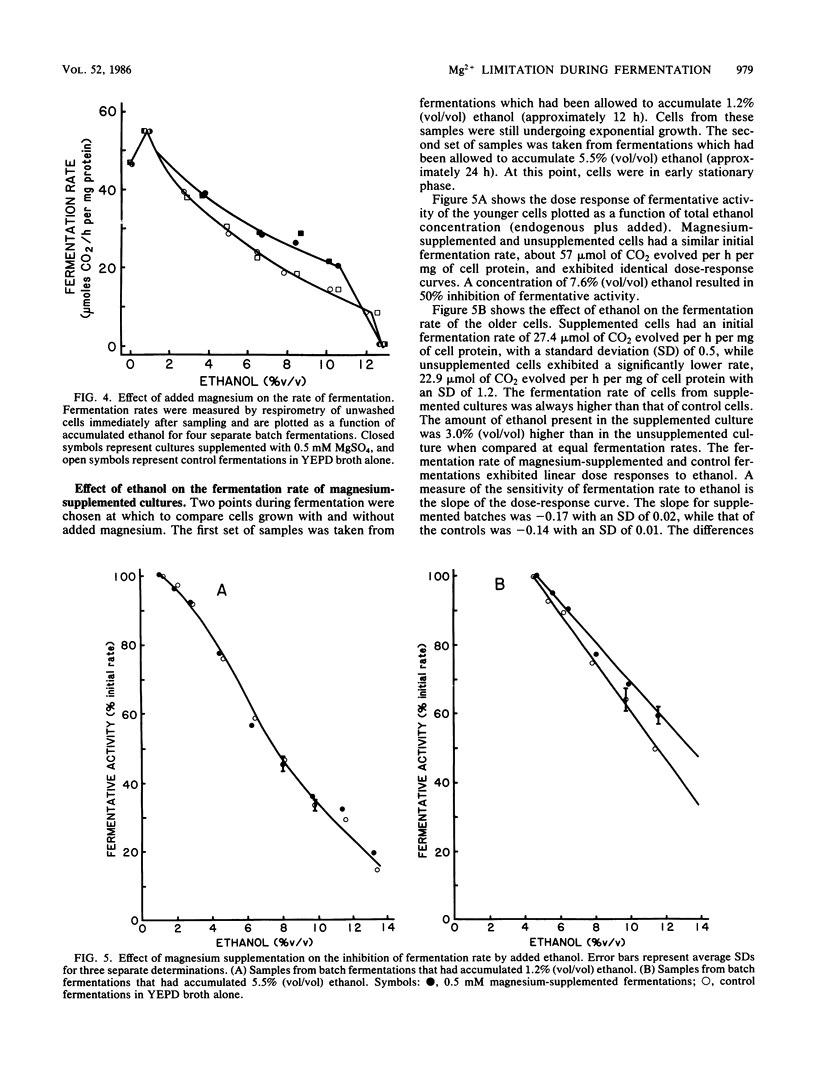
The rate of ethanol production per milligram of cell protein begins to decline in the early stage of batch fermentation before high concentrations of ethanol have accumulated. In yeast extract-peptone medium (20% glucose), this initial decline appears to be related to growth and to result in part from a nutrient deficiency. The addition of yeast extract, peptone, and ashed preparations of these restored the ability of glucose-reconstituted medium (in which cells had been previously grown) to support vigorous growth. Magnesium was identified as the active component. Supplementing fermentations with 0.5 mM magnesium prolonged exponential growth, resulting in increased yeast cell mass. The addition of magnesium also reduced the decline in fermentative activity (micromoles of CO2 evolved per hour per milligram of protein) during the completion of batch fermentations. These two effects reduced the time required for the conversion of 20% glucose into ethanol by 1/3 with no measurable loss in ethanol yield (98% of theoretical maximum yield). It is possible that some of the reported beneficial effects of complex nutrients (soy flour and yeast extract) for ethanol production also result from the correction of a simple inorganic ion deficiency, such as magnesium.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazua C. D., Wilke C. R. Ethanol effects on the kinetics of a continuous fermentation with Saccharomyces cerevisiae. Biotechnol Bioeng Symp. 1977;(7):105–118. [PubMed] [Google Scholar]
- Casey G. P., Magnus C. A., Ingledew W. M. High-gravity brewing: effects of nutrition on yeast composition, fermentative ability, and alcohol production. Appl Environ Microbiol. 1984 Sep;48(3):639–646. doi: 10.1128/aem.48.3.639-646.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombek K. M., Ingram L. O. Determination of the intracellular concentration of ethanol in Saccharomyces cerevisiae during fermentation. Appl Environ Microbiol. 1986 Jan;51(1):197–200. doi: 10.1128/aem.51.1.197-200.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray W. D. Studies on the Alcohol Tolerance of Yeasts. J Bacteriol. 1941 Nov;42(5):561–574. doi: 10.1128/jb.42.5.561-574.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guijarro J. M., Lagunas R. Saccharomyces cerevisiae does not accumulate ethanol against a concentration gradient. J Bacteriol. 1984 Dec;160(3):874–878. doi: 10.1128/jb.160.3.874-878.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossack J. A., Rose A. H. Fragility of plasma membranes in Saccharomyces cerevisiae enriched with different sterols. J Bacteriol. 1976 Jul;127(1):67–75. doi: 10.1128/jb.127.1.67-75.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O., Buttke T. M. Effects of alcohols on micro-organisms. Adv Microb Physiol. 1984;25:253–300. doi: 10.1016/s0065-2911(08)60294-5. [DOI] [PubMed] [Google Scholar]
- Janssens J. H., Burris N., Woodward A., Bailey R. B. Lipid-Enhanced Ethanol Production by Kluyveromyces fragilis. Appl Environ Microbiol. 1983 Feb;45(2):598–602. doi: 10.1128/aem.45.2.598-602.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafon-Lafourcade S., Larue F., Ribereau-Gayon P. Evidence for the existence of "survival factors" as an explanation for some peculiarities of yeast growth, especially in grape must of high sugar concentration. Appl Environ Microbiol. 1979 Dec;38(6):1069–1073. doi: 10.1128/aem.38.6.1069-1073.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagodawithana T. W., Steinkraus K. H. Influence of the rate of ethanol production and accumulation on the viability of Saccharomyces cerevisiae in "rapid fermentation". Appl Environ Microbiol. 1976 Feb;31(2):158–162. doi: 10.1128/aem.31.2.158-162.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro J. M., Durand G. Fermentation alcoolique: influence de la température sur l'accumulation d'alcool dans les cellules de levure. Ann Microbiol (Paris) 1978 Aug-Sep;129B(2):215–224. [PubMed] [Google Scholar]
- Nes W. R., Sekula B. C., Nes W. D., Adler J. H. The functional importance of structural features of ergosterol in yeast. J Biol Chem. 1978 Sep 10;253(17):6218–6225. [PubMed] [Google Scholar]
- Ohta K., Hayashida S. Role of tween 80 and monoolein in a lipid-sterol-protein complex which enhances ethanol tolerance of sake yeasts. Appl Environ Microbiol. 1983 Oct;46(4):821–825. doi: 10.1128/aem.46.4.821-825.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman Y. A., Ingram L. O. Mechanism of ethanol inhibition of fermentation in Zymomonas mobilis CP4. J Bacteriol. 1985 Oct;164(1):173–180. doi: 10.1128/jb.164.1.173-180.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudlock J. W., Wheeldon L. W., Jollow D. J., Linnane A. W. Role of sterols in Saccharomyces cerevisiae. Biochim Biophys Acta. 1968 Mar 4;152(2):434–437. doi: 10.1016/0005-2760(68)90060-x. [DOI] [PubMed] [Google Scholar]
- Rahn O. THE DECREASING RATE OF FERMENTATION. J Bacteriol. 1929 Sep;18(3):207–226. doi: 10.1128/jb.18.3.207-226.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. S., Hossack J. A., Rose A. H. Plasma-membrane lipid composition and ethanol tolerance in Saccharomyces cerevisiae. Arch Microbiol. 1978 Jun 26;117(3):239–245. doi: 10.1007/BF00738541. [DOI] [PubMed] [Google Scholar]
- Viegas C. A., Sá-Correia I., Novais J. M. Nutrient-Enhanced Production of Remarkably High Concentrations of Ethanol by Saccharomyces bayanus through Soy Flour Supplementation. Appl Environ Microbiol. 1985 Nov;50(5):1333–1335. doi: 10.1128/aem.50.5.1333-1335.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. M., Duffus J. H. Magnesium ions and the control of the cell cycle in yeast. J Cell Sci. 1980 Apr;42:329–356. doi: 10.1242/jcs.42.1.329. [DOI] [PubMed] [Google Scholar]


