Abstract
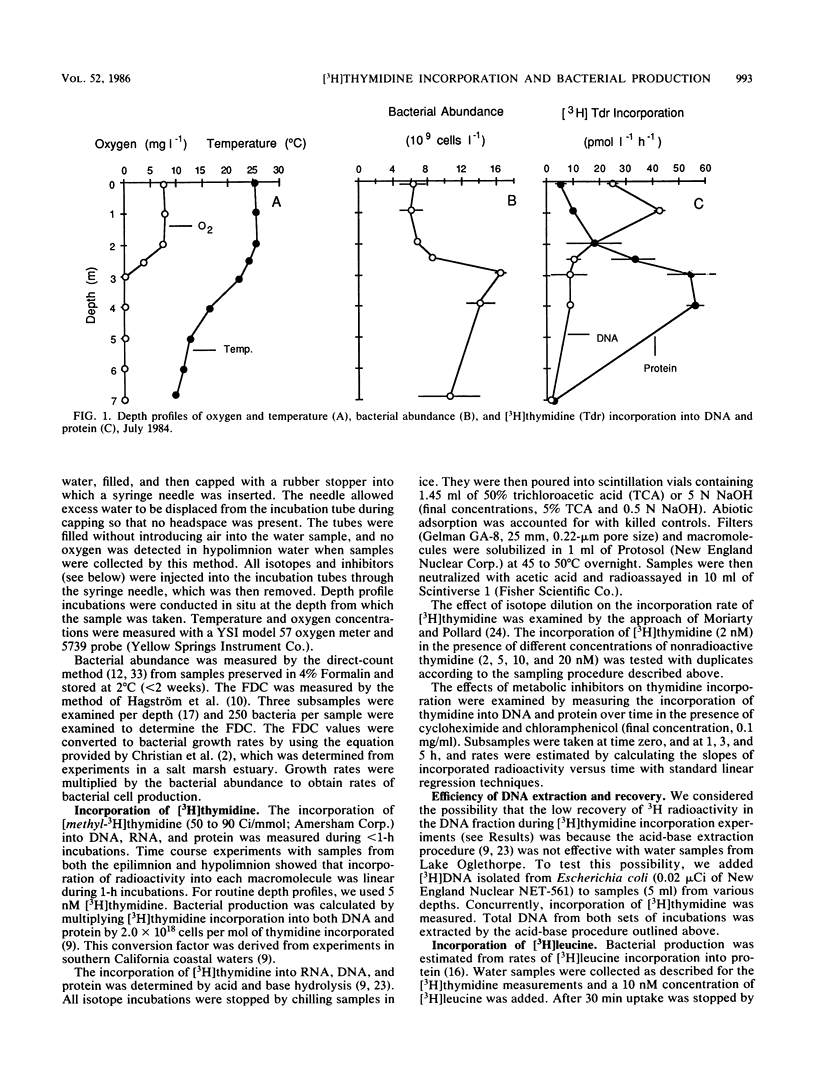
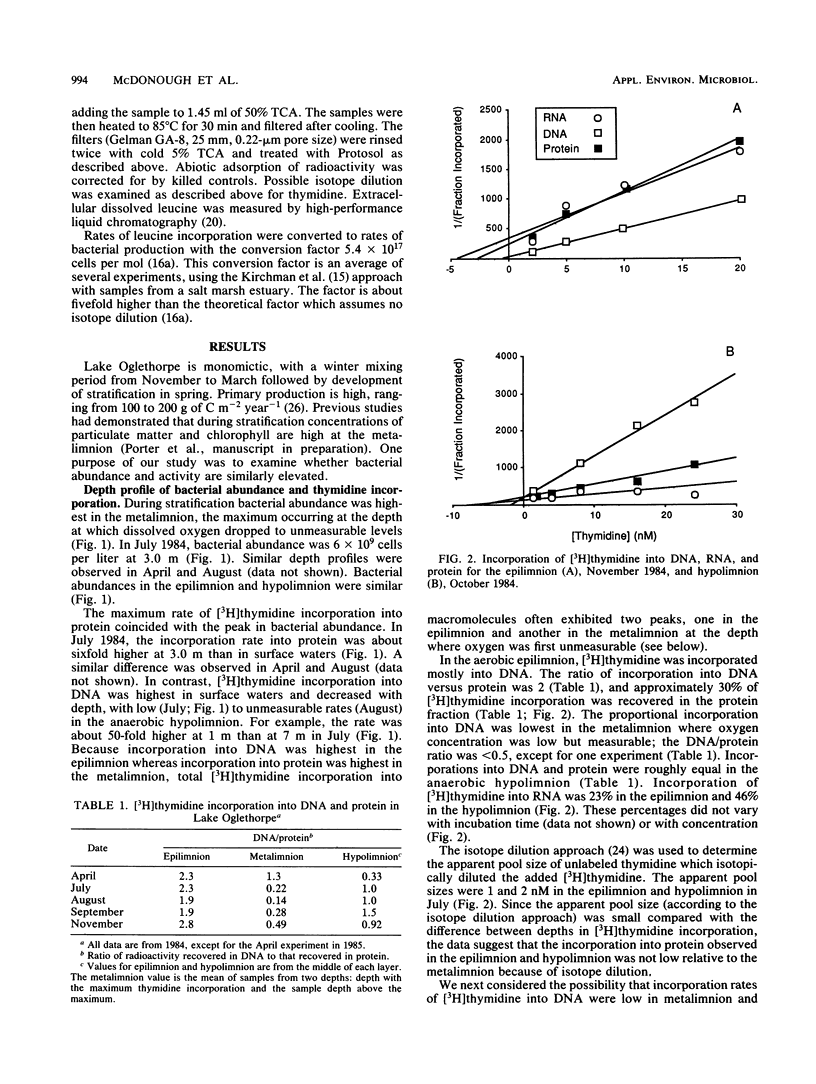
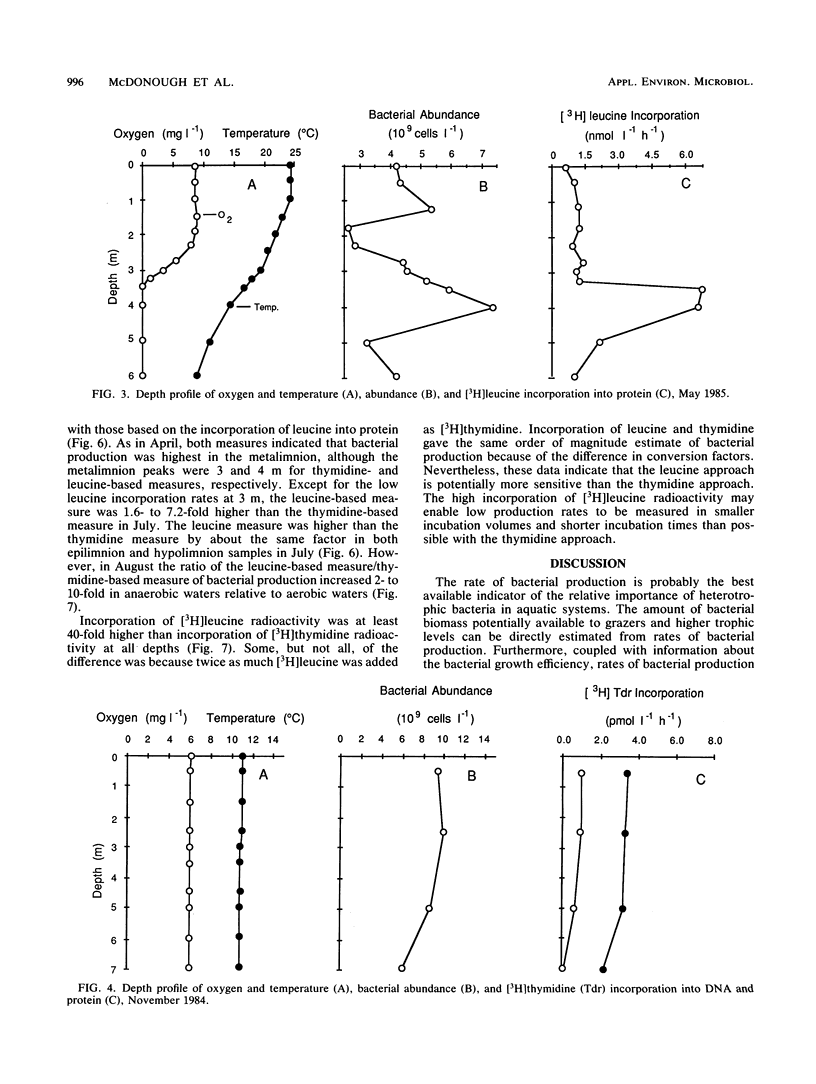
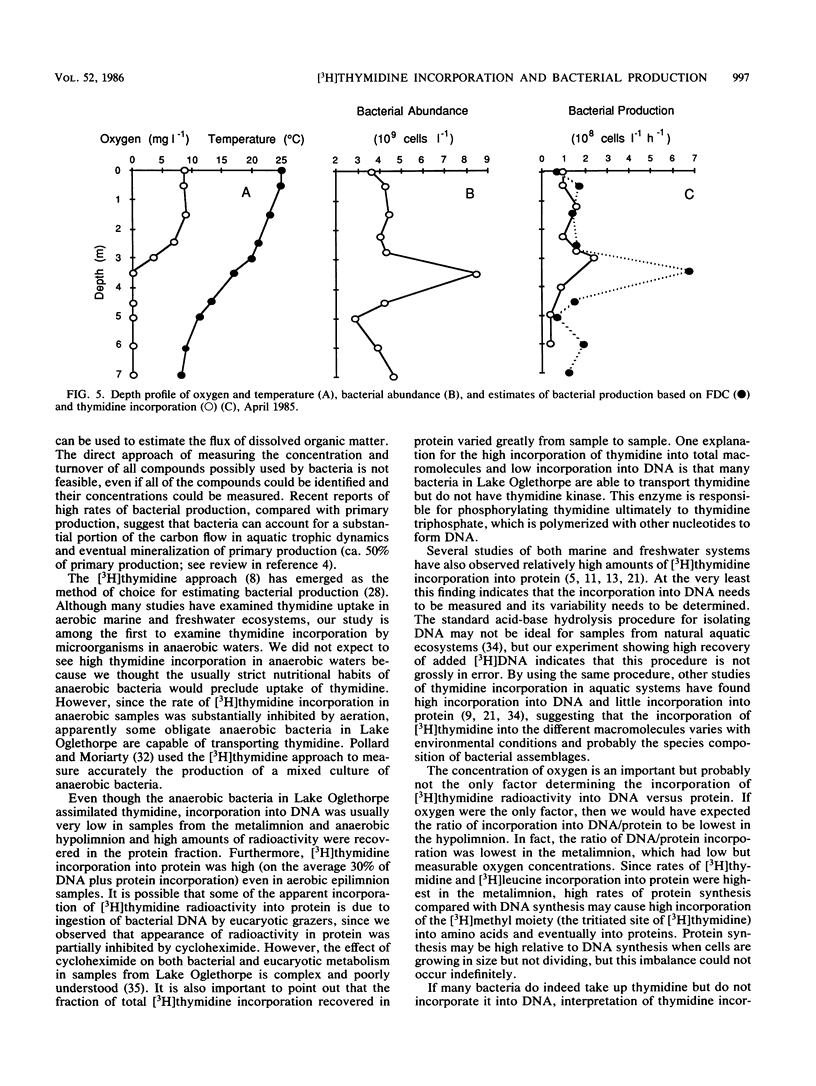
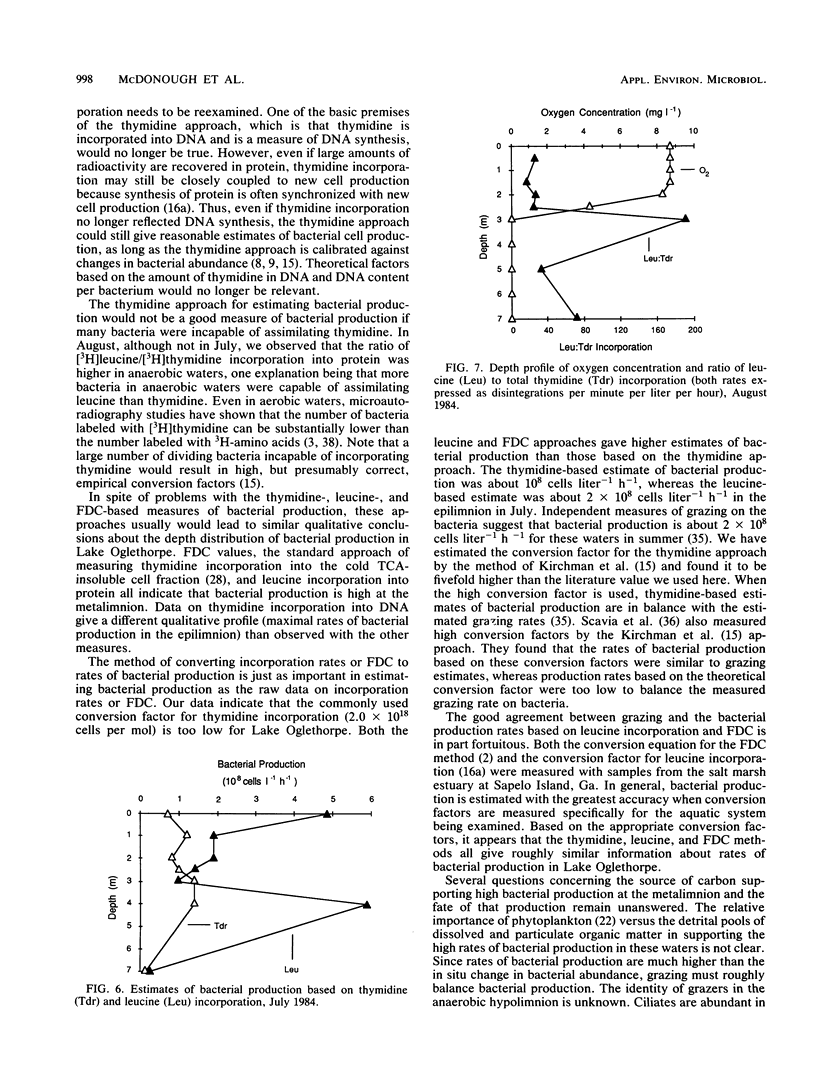
The purpose of this study was to determine the depth distribution of bacterial biomass and production in a stratified lake and to test techniques to measure bacterial production in anaerobic waters. Bacterial abundance and incorporation of both [3H]thymidine and [3H]leucine into protein were highest in the metalimnion, at the depth at which oxygen first became unmeasurable. In contrast, [3H]thymidine incorporation into DNA was highest in the epilimnion. The ratios of incorporation into DNA/protein averaged 2.2, 0.49, and 0.95 for the epilimnion, metalimnion, and hypolimnion, respectively. Low incorporation into DNA was not due to artifacts associated with the DNA isolation procedure. Recovery of added [3H]DNA was about 90% in waters in which the portion of [3H]thymidine incorporation into DNA was about 40%. At least some obligate anaerobic bacteria were capable of assimilating thymidine since aeration of anaerobic hypolimnion waters substantially inhibited thymidine incorporation. The depth profile of bacterial production estimated from total thymidine and leucine incorporation and the frequency of dividing cells were all similar, with maximal rates in the metalimnion. However, estimates of bacterial production based on frequency of dividing cells and leucine incorporation were usually significantly higher than estimates based on thymidine incorporation (using conversion factors from the literature), especially in anaerobic hypolimnion waters. These data indicate that the thymidine approach must be examined carefully if it is to be applied to aquatic systems with low oxygen concentrations. Our results also indicate that the interface between the aerobic epilimnion and anaerobic hypolimnion is the site of intense bacterial mineralization and biomass production which deserves further study.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christian R. R., Hanson R. B., Newell S. Y. Comparison of methods for measurement of bacterial growth rates in mixed batch cultures. Appl Environ Microbiol. 1982 May;43(5):1160–1165. doi: 10.1128/aem.43.5.1160-1165.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. A., Azam F. Bacterioplankton secondary production estimates for coastal waters of british columbia, antarctica, and california. Appl Environ Microbiol. 1980 Jun;39(6):1085–1095. doi: 10.1128/aem.39.6.1085-1095.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagström A., Larsson U., Hörstedt P., Normark S. Frequency of dividing cells, a new approach to the determination of bacterial growth rates in aquatic environments. Appl Environ Microbiol. 1979 May;37(5):805–812. doi: 10.1128/aem.37.5.805-812.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., Ducklow H., Mitchell R. Estimates of bacterial growth from changes in uptake rates and biomass. Appl Environ Microbiol. 1982 Dec;44(6):1296–1307. doi: 10.1128/aem.44.6.1296-1307.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., Hodson R. Inhibition by peptides of amino Acid uptake by bacterial populations in natural waters: implications for the regulation of amino Acid transport and incorporation. Appl Environ Microbiol. 1984 Apr;47(4):624–631. doi: 10.1128/aem.47.4.624-631.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., K'nees E., Hodson R. Leucine incorporation and its potential as a measure of protein synthesis by bacteria in natural aquatic systems. Appl Environ Microbiol. 1985 Mar;49(3):599–607. doi: 10.1128/aem.49.3.599-607.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., Sigda J., Kapuscinski R., Mitchell R. Statistical analysis of the direct count method for enumerating bacteria. Appl Environ Microbiol. 1982 Aug;44(2):376–382. doi: 10.1128/aem.44.2.376-382.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell C. R., Konopka A. Primary and bacterial production in two dimictic indiana lakes. Appl Environ Microbiol. 1985 Mar;49(3):485–491. doi: 10.1128/aem.49.3.485-491.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrós-Alió C., Brock T. D. Assessing biomass and production of bacteria in eutrophic lake mendota, wisconsin. Appl Environ Microbiol. 1982 Jul;44(1):203–218. doi: 10.1128/aem.44.1.203-218.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard P. C., Moriarty D. J. Validity of the tritiated thymidine method for estimating bacterial growth rates: measurement of isotope dilution during DNA synthesis. Appl Environ Microbiol. 1984 Dec;48(6):1076–1083. doi: 10.1128/aem.48.6.1076-1083.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor P. S., Neihof R. A. Direct determination of activities for microorganisms of chesapeake bay populations. Appl Environ Microbiol. 1984 Nov;48(5):1012–1019. doi: 10.1128/aem.48.5.1012-1019.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


