Abstract
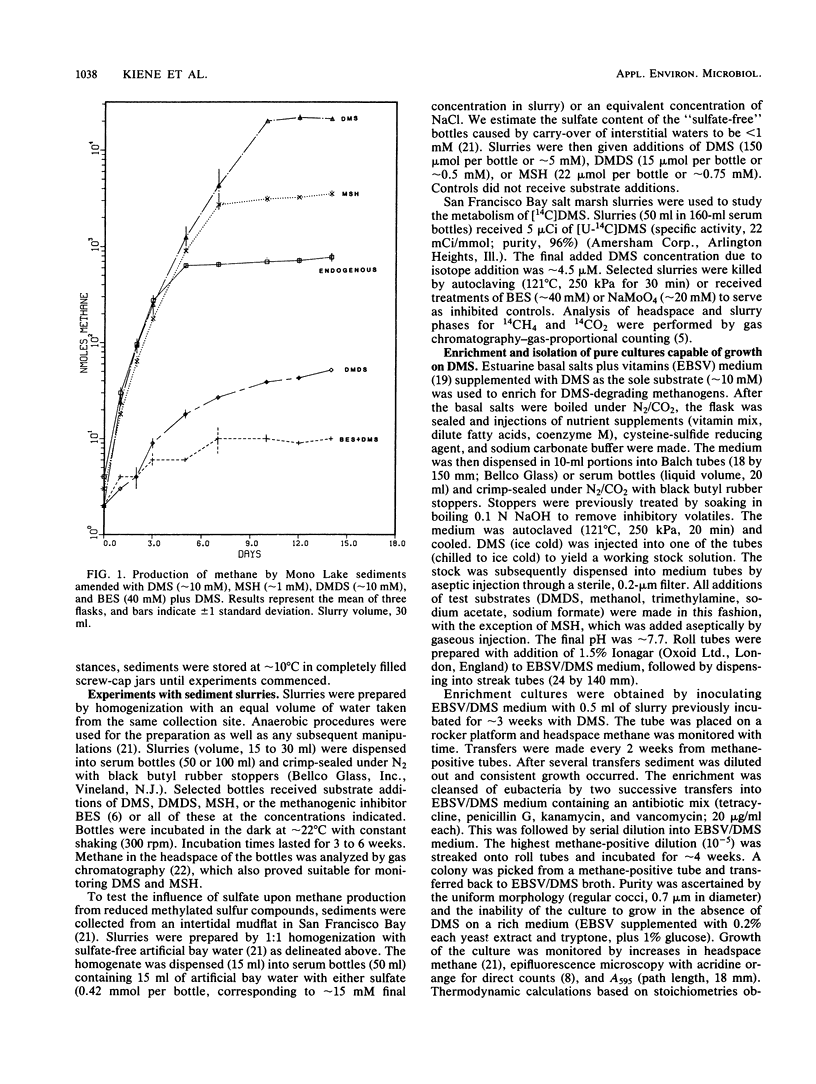
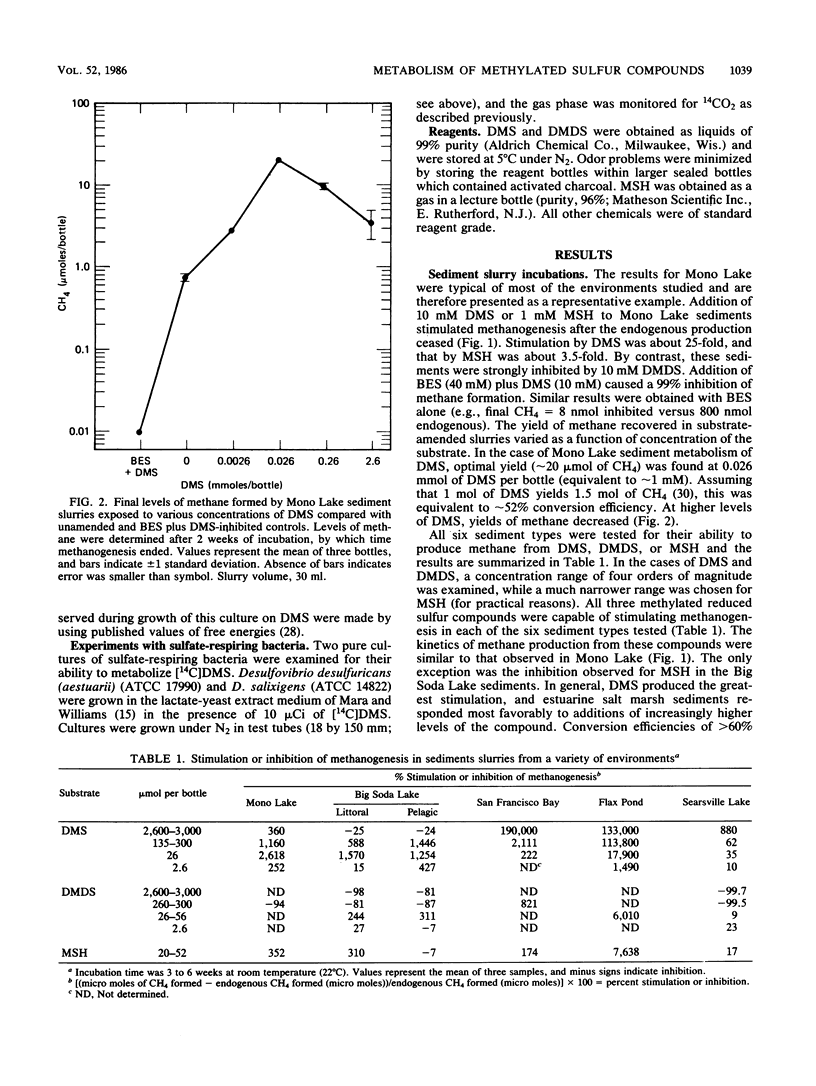
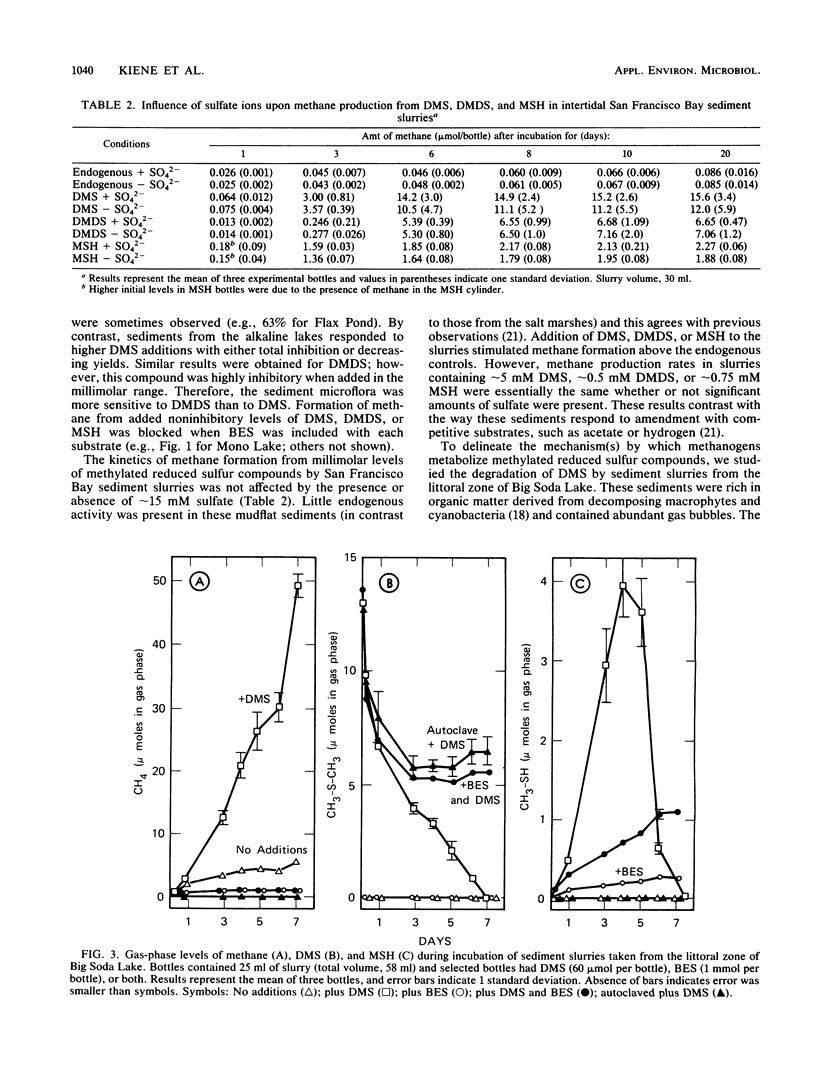
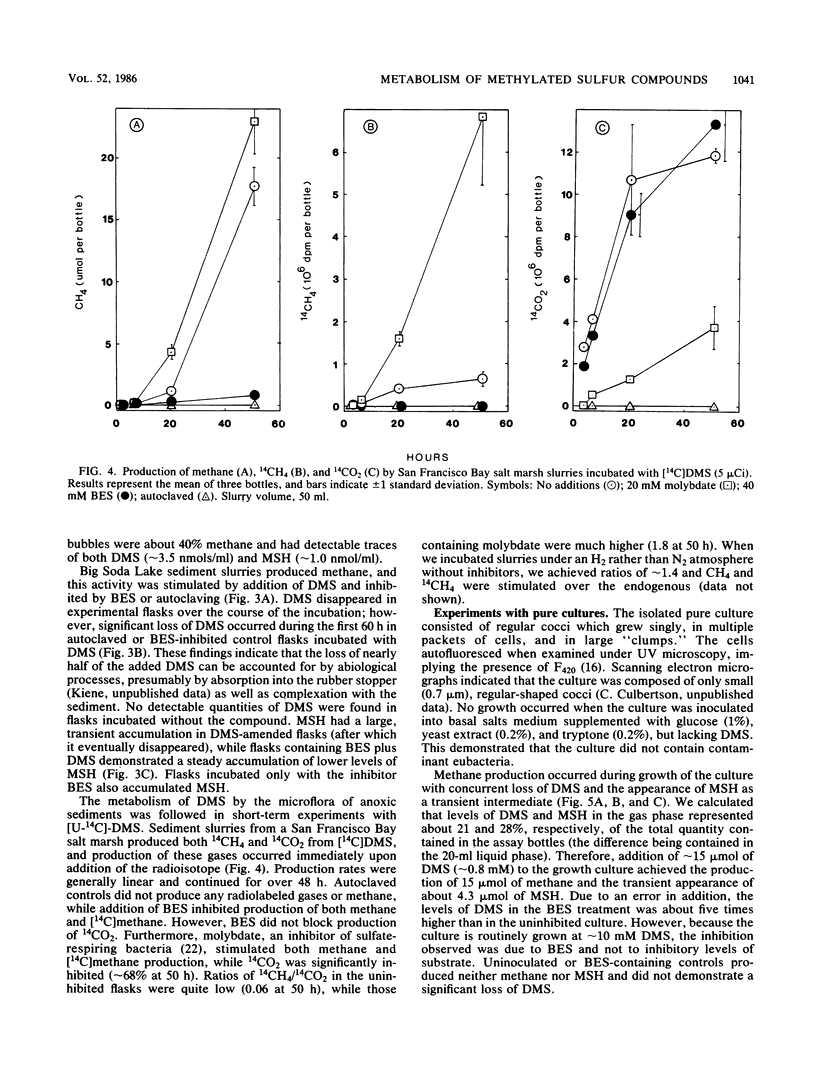
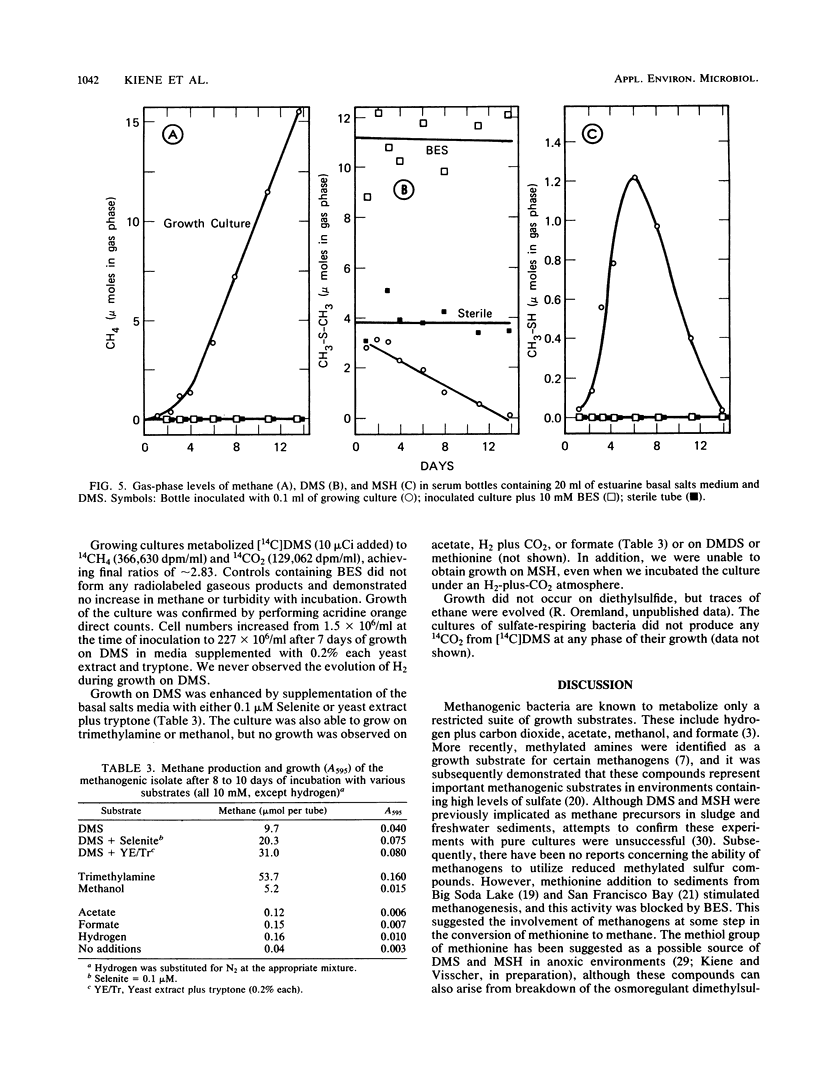
Addition of dimethylsulfide (DMS), dimethyldisulfide (DMDS), or methane thiol (MSH) to a diversity of anoxic aquatic sediments (e.g., fresh water, estuarine, alkaline/hypersaline) stimulated methane production. The yield of methane recovered from DMS was often 52 to 63%, although high concentrations of DMS (as well as MSH and DMDS) inhibited methanogenesis in some types of sediments. Production of methane from these reduced methylated sulfur compounds was blocked by 2-bromoethanesulfonic acid. Sulfate did not influence the metabolism of millimolar levels of DMS, DMDS, or MSH added to sediments. However, when DMS was added at ∼2-μM levels as [14C]DMS, metabolism by sediments resulted in a 14CH4/14CO2 ratio of only 0.06. Addition of molybdate increased the ratio to 1.8, while 2-bromoethanesulfonic acid decreased it to 0, but did not block 14CO2 production. These results indicate the methanogens and sulfate reducers compete for DMS when it is present at low concentrations; however, at high concentrations, DMS is a “noncompetitive” substrate for methanogens. Metabolism of DMS by sediments resulted in the appearance of MSH as a transient intermediate. A pure culture of an obligately methylotrophic estuarine methanogen was isolated which was capable of growth on DMS. Metabolism of DMS by the culture also resulted in the transient appearance of MSH, but the organism could grow on neither MSH nor DMDS. The culture metabolized [14C]-DMS to yield a 14CH4/14CO2 ratio of ∼2.8. Reduced methylated sulfur compounds represent a new class of substrates for methanogens and may be potential precursors of methane in a variety of aquatic habitats.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreae M. O., Raemdonck H. Dimethyl sulfide in the surface ocean and the marine atmosphere: a global view. Science. 1983 Aug 19;221(4612):744–747. doi: 10.1126/science.221.4612.744. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson C. W., Zehnder A. J., Oremland R. S. Anaerobic oxidation of acetylene by estuarine sediments and enrichment cultures. Appl Environ Microbiol. 1981 Feb;41(2):396–403. doi: 10.1128/aem.41.2.396-403.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Romesser J. A., Wolfe R. S. Preparation of coenzyme M analogues and their activity in the methyl coenzyme M reductase system of Methanobacterium thermoautotrophicum. Biochemistry. 1978 Jun 13;17(12):2374–2377. doi: 10.1021/bi00605a019. [DOI] [PubMed] [Google Scholar]
- Hippe H., Caspari D., Fiebig K., Gottschalk G. Utilization of trimethylamine and other N-methyl compounds for growth and methane formation by Methanosarcina barkeri. Proc Natl Acad Sci U S A. 1979 Jan;76(1):494–498. doi: 10.1073/pnas.76.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. B., Stadtman T. C. Methanococcus vannielii: culture and effects of selenium and tungsten on growth. J Bacteriol. 1977 Jun;130(3):1404–1406. doi: 10.1128/jb.130.3.1404-1406.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mara D. D., Williams D. J. The evaluation of media used to enumerate sulphate reducing bacteria. J Appl Bacteriol. 1970 Sep;33(3):543–552. doi: 10.1111/j.1365-2672.1970.tb02232.x. [DOI] [PubMed] [Google Scholar]
- Mink R. W., Dugan P. R. Tentative identification of methanogenic bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 Mar;33(3):713–717. doi: 10.1128/aem.33.3.713-717.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S. Hydrogen metabolism by decomposing cyanobacterial aggregates in big soda lake, nevada. Appl Environ Microbiol. 1983 May;45(5):1519–1525. doi: 10.1128/aem.45.5.1519-1525.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Marsh L., Desmarais D. J. Methanogenesis in big soda lake, nevada: an alkaline, moderately hypersaline desert lake. Appl Environ Microbiol. 1982 Feb;43(2):462–468. doi: 10.1128/aem.43.2.462-468.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S. Microbial formation of ethane in anoxic estuarine sediments. Appl Environ Microbiol. 1981 Jul;42(1):122–129. doi: 10.1128/aem.42.1.122-129.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Polcin S. Methanogenesis and sulfate reduction: competitive and noncompetitive substrates in estuarine sediments. Appl Environ Microbiol. 1982 Dec;44(6):1270–1276. doi: 10.1128/aem.44.6.1270-1276.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers K. R., Ferry J. G. Isolation and Characterization of a Methylotrophic Marine Methanogen, Methanococcoides methylutens gen. nov., sp. nov. Appl Environ Microbiol. 1983 Feb;45(2):684–690. doi: 10.1128/aem.45.2.684-690.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder S. H., Brock T. D. Methane, carbon dioxide, and hydrogen sulfide production from the terminal methiol group of methionine by anaerobic lake sediments. Appl Environ Microbiol. 1978 Feb;35(2):344–352. doi: 10.1128/aem.35.2.344-352.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder S. H., Doemel W. N., Brock T. D. Production of volatile sulfur compounds during the decomposition of algal mats. Appl Environ Microbiol. 1977 Dec;34(6):859–860. doi: 10.1128/aem.34.6.859-860.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



