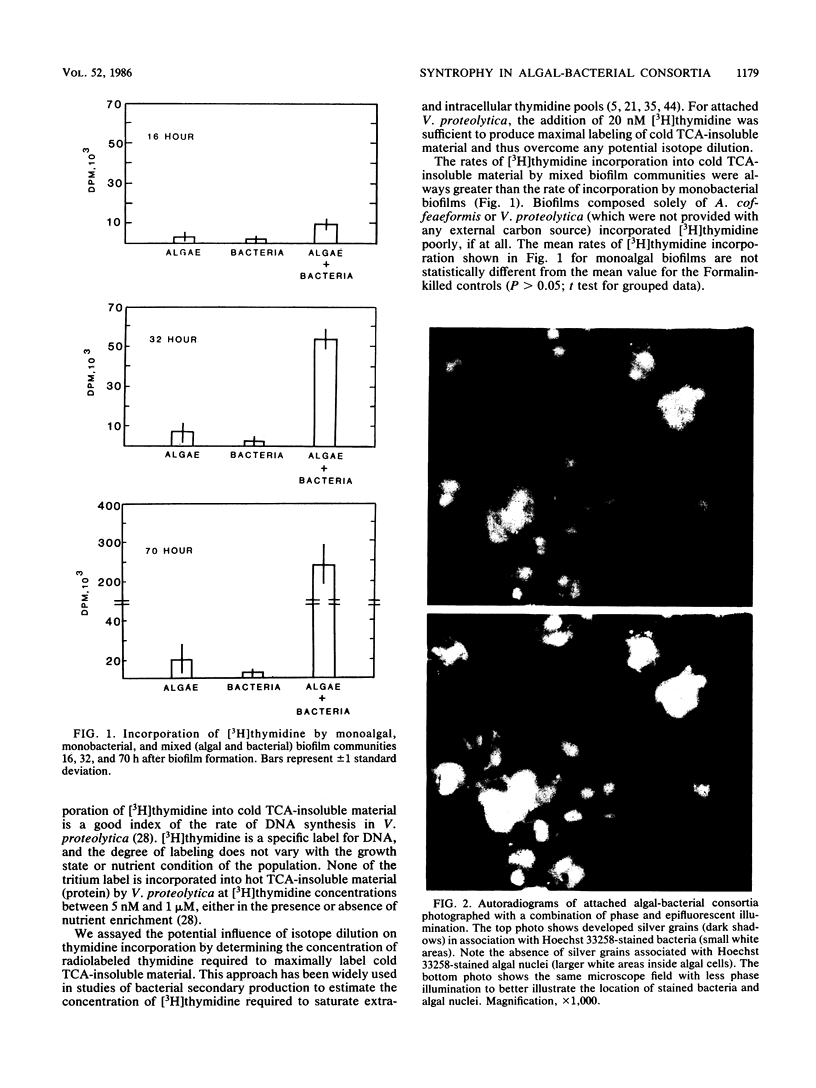
Abstract
Algal-bacterial consortia attached to polystyrene surfaces were prepared in the laboratory by using the marine diatom Amphora coffeaeformis and the marine bacterium Vibrio proteolytica (the approved name of this bacterium is Vibrio proteolyticus [W. E. C. Moore, E. P. Cato, and L. V. H. Moore, Int. J. Syst. Bacteriol. 35:382-407, 1985]). The organisms were attached to the surfaces at cell densities of approximately 5 × 104 cells cm-2 (diatoms) and 5 × 106 cells cm-2 (bacteria). The algal-bacterial consortia consistently exhibited higher rates of [3H]thymidine incorporation than did biofilms composed solely of bacteria. The rates of [3H]thymidine incorporation by the algal-bacterial consortia were fourfold greater than the rates of incorporation by monobacterial biofilms 16 h after biofilm formation and were 16-fold greater 70 h after biofilm formation. Extracellular material released from the attached Amphora cells supported rates of bacterial activity (0.8 × 10-21 to 17.9 × 10-21 mol of [3H]thymidine incorporated cell-1 h-1) and growth (doubling time, 29.5 to 1.4 days) comparable to values reported for a wide variety of marine and freshwater ecosystems. In the presence of sessile diatom populations, DNA synthesis by attached V. proteolytica cells was light dependent and increased with increasing algal abundance. The metabolic activity of diatoms thus appears to be the rate-limiting process in biofilm development on illuminated surfaces under conditions of low bulk-water dissolved organic carbon.
Full text
PDF

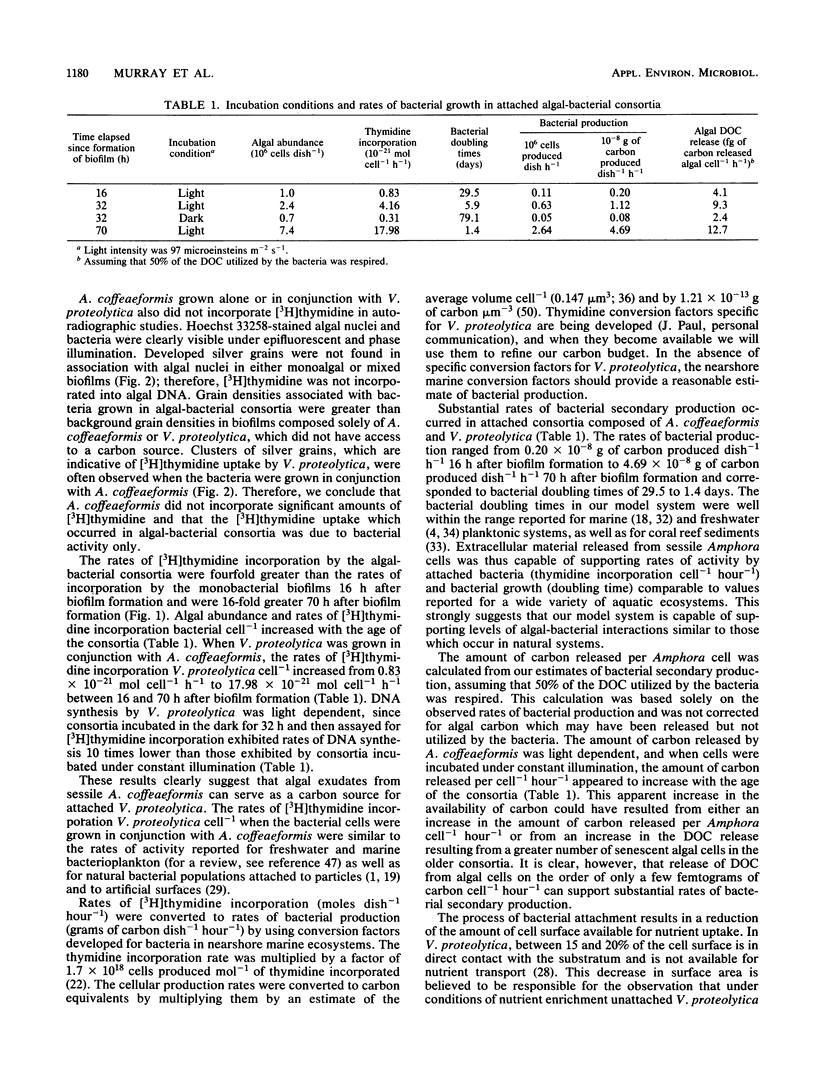


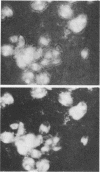
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. T., Ahlgren G. M., Ahlgren I. Estimating Bacterioplankton Production by Measuring [H]thymidine Incorporation in a Eutrophic Swedish Lake. Appl Environ Microbiol. 1983 Jun;45(6):1709–1721. doi: 10.1128/aem.45.6.1709-1721.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D., Clyne J. Significance of algal excretory products for growth of epilimnetic bacteria. Appl Environ Microbiol. 1984 Apr;47(4):731–734. doi: 10.1128/aem.47.4.731-734.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooksey K. E. Requirement for calcium in adhesion of a fouling diatom to glass. Appl Environ Microbiol. 1981 Jun;41(6):1378–1382. doi: 10.1128/aem.41.6.1378-1382.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducklow H. W., Kirchman D. L., Rowe G. T. Production and vertical flux of attached bacteria in the hudson river plume of the new york bight as studied with floating sediment traps. Appl Environ Microbiol. 1982 Apr;43(4):769–776. doi: 10.1128/aem.43.4.769-776.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducklow H. W., Purdie D. A., Williams P. J., Davies J. M. Bacterioplankton: a sink for carbon in a coastal marine plankton community. Science. 1986 May 16;232(4752):865–867. doi: 10.1126/science.232.4752.865. [DOI] [PubMed] [Google Scholar]
- Fuhrman J. A., Azam F. Bacterioplankton secondary production estimates for coastal waters of british columbia, antarctica, and california. Appl Environ Microbiol. 1980 Jun;39(6):1085–1095. doi: 10.1128/aem.39.6.1085-1095.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack T. K., McFeters G. A. Microbial dynamics of an epilithic mat community in a high alpine stream. Appl Environ Microbiol. 1982 Mar;43(3):702–707. doi: 10.1128/aem.43.3.702-707.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey W. H., Paul J. H. Activity measurements of planktonic microbial and microfouling communities in a eutrophic estuary. Appl Environ Microbiol. 1986 Jan;51(1):157–162. doi: 10.1128/aem.51.1.157-162.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey W. H., Paul J. H. Activity of an Attached and Free-Living Vibrio sp. as Measured by Thymidine Incorporation, p-Iodonitrotetrazolium Reduction, and ATP/DNA Ratios. Appl Environ Microbiol. 1986 Jan;51(1):150–156. doi: 10.1128/aem.51.1.150-156.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. E., Hodson R. E. Annual cycle of bacterial secondary production in five aquatic habitats of the okefenokee swamp ecosystem. Appl Environ Microbiol. 1985 Mar;49(3):650–655. doi: 10.1128/aem.49.3.650-655.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. E., Hodson R. E. Influence of macrophyte decomposition on growth rate and community structure of okefenokee swamp bacterioplankton. Appl Environ Microbiol. 1986 Feb;51(2):293–301. doi: 10.1128/aem.51.2.293-301.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. H. Effects of antimetabolites on the adhesion of an estuarine Vibrio sp. to polystyrene. Appl Environ Microbiol. 1984 Nov;48(5):924–929. doi: 10.1128/aem.48.5.924-929.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. H., Jeffrey W. H. Evidence for Separate Adhesion Mechanisms for Hydrophilic and Hydrophobic Surfaces in Vibrio proteolytica. Appl Environ Microbiol. 1985 Aug;50(2):431–437. doi: 10.1128/aem.50.2.431-437.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. H., Loeb G. I. Improved Microfouling Assay Employing a DNA-Specific Fluorochrome and Polystyrene as Substratum. Appl Environ Microbiol. 1983 Aug;46(2):338–343. doi: 10.1128/aem.46.2.338-343.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. H. Use of hoechst dyes 33258 and 33342 for enumeration of attached and planktonic bacteria. Appl Environ Microbiol. 1982 Apr;43(4):939–944. doi: 10.1128/aem.43.4.939-944.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson B. J., Hobbie J. E., Hershey A. E., Lock M. A., Ford T. E., Vestal J. R., McKinley V. L., Hullar M. A., Miller M. C., Ventullo R. M., Volk G. S. Transformation of a tundra river from heterotrophy to autotrophy by addition of phosphorus. Science. 1985 Sep 27;229(4720):1383–1386. doi: 10.1126/science.229.4720.1383. [DOI] [PubMed] [Google Scholar]
- Staley J. T., Konopka A. Measurement of in situ activities of nonphotosynthetic microorganisms in aquatic and terrestrial habitats. Annu Rev Microbiol. 1985;39:321–346. doi: 10.1146/annurev.mi.39.100185.001541. [DOI] [PubMed] [Google Scholar]
- Tison D. L., Pope D. H., Cherry W. B., Fliermans C. B. Growth of Legionella pneumophila in association with blue-green algae (cyanobacteria). Appl Environ Microbiol. 1980 Feb;39(2):456–459. doi: 10.1128/aem.39.2.456-459.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. W., Novitsky T. J., Quinby H. L., Valois F. W. Determination of bacterial number and biomass in the marine environment. Appl Environ Microbiol. 1977 Apr;33(4):940–946. doi: 10.1128/aem.33.4.940-946.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]