Abstract
Erythropoietin (Epo)-independent differentiation of erythroid progenitors is a major characteristic of myeloproliferative disorders, including chronic myeloid leukemia. Epo receptor (EpoR) signaling is crucial for normal erythroid development, as evidenced by the properties of Epo−/− and EpoR−/− mice, which contain a normal number of fetal liver erythroid progenitors but die in utero from a severe anemia attributable to the absence of red cell maturation. Here we show that two constitutively active cytoplasmic protein tyrosine kinases, P210BCR-ABL and v-SRC, can functionally replace the EpoR and support full proliferation, differentiation, and maturation of fetal liver erythroid progenitors from EpoR−/− mice. These protein tyrosine kinases can also partially complement the myeloid growth factors IL-3, IL-6, and Steel factor, which are normally required in addition to Epo for erythroid development. Additionally, BCR-ABL mutants that lack residues necessary for transformation of fibroblasts or bone marrow cells can fully support normal erythroid development. These results demonstrate that activated tyrosine kinase oncoproteins implicated in tumorigenesis and human leukemia can functionally complement for cytokine receptor signaling pathways to support normal erythropoiesis in EpoR-deficient cells. Moreover, terminal differentiation of erythroid cells requires generic signals provided by activated protein tyrosine kinases and does not require a specific signal unique to a cytokine receptor.
Erythropoietin (EpoR) signaling is crucial for erythroid development. During days 11–14 of gestation, the embryonic liver becomes the major site of erythropoiesis. Disruption of either Epo or the Epo receptor (EpoR) genes in mice leads to embryonic lethality at around days 13–15 (1–3) because of deficiency in definitive erythropoiesis and severe fetal anemia. Fetal livers of Epo−/− or EpoR−/− mice contain normal numbers of primitive burst-forming-unit (BFU-E) and mature colony-forming-unit (CFU-E) erythroid progenitors, but these fail to differentiate into red blood cells in the absence of EpoR signaling (1). Retroviral transduction of the EpoR into EpoR−/− fetal liver progenitors rescues normal Epo-dependent erythropoiesis, demonstrating the essential requirement for EpoR signaling during differentiation of committed BFU-E and CFU-E progenitors (1).
Epo-independent proliferation and differentiation of erythroid progenitors in vitro is a property common to all myeloproliferative disorders and serves as a diagnostic criterion (4). Chronic myeloid leukemia (CML) is a biphasic, multilineage, and clonal myeloproliferative disorder caused by a reciprocal chromosomal translocation t (9:22) (5) that fuses 5′ BCR gene sequences upstream of the second c-ABL exon, resulting in the formation of the fusion oncogene BCR-ABL (6). The product of this gene is a chimeric 210-kDa protein (P210BCR-ABL) expressed in all hematopoietic cells of CML patients. The P210 protein is a constitutively active tyrosine kinase that is significantly more active than the nuclear c-ABL protein tyrosine kinase (7, 8). Expression of P210BCR-ABL in a primitive hematopoietic stem cell (9) results in the initial chronic phase of CML, characterized by an expansion of the myeloid hematopoietic compartment that leads clinically to increased numbers of well differentiated granulocytes and often massive splenomegaly (10, 11). Expression of P210BCR-ABL in established myeloid cell lines confers cytokine-independent proliferation. Classic studies of primary bone marrow from CML patients failed to demonstrate any abrogation of growth factor requirement for granulocytic-macrophage progenitors in vitro (12), but more recent analysis of the most primitive progenitors suggests that these early cells indeed proliferate and differentiate in the absence of added cytokines (13). Erythroid progenitors are also increased in the peripheral blood of CML patients. Although CML granulocytic progenitors require cytokines for proliferation and differentiation in vitro, CML erythroid progenitors can proliferate and differentiate in cultures supplemented with Steel factor (SF) in the absence of Epo or any other cytokines (4, 14). Given the Epo-independence of CML erythroid progenitors, we reasoned that BCR-ABL might be complementing for EpoR signaling.
The EpoR activates a number of signaling proteins and pathways, including JAK2, STAT5, PI3-kinase, Ras MAPK, SHP1 and SHP2, SHIP, Bcl-2, Bcl-X and AKT (reviewed in ref. 15). Most, if not all, of these proteins are also activated by BCR-ABL (reviewed in ref. 16). A constitutively active tyrosine kinase is crucial for transformation by BCR-ABL (8, 17). BCR-ABL domains such as the oligomerization region in the N terminus or the SH3 domain are thought to regulate the activity of the tyrosine kinase and also affect transformation by BCR-ABL (18–20). Critical residues of BCR-ABL, including tyrosine 177 (Y177) and the SH2 domain, do not regulate BCR-ABL tyrosine kinase activity but nevertheless impact cellular transformation by BCR-ABL (21, 22). Phosphorylated tyrosine 177 binds Grb-2 and couples BCR-ABL to activation of the Ras pathway (23, 24); Y177 is essential for transformation of fibroblasts and bone marrow cells by BCR-ABL (23) but has been reported to be dispensable for tumorigenesis in vivo (22). The ABL SH2 domain within BCR-ABL binds phosphotyrosine residues of several proteins and is also required for BCR-ABL transformation of fibroblasts and leukemogenesis in vivo (21, 22).
The highly transforming v-SRC protein of chicken Rous sarcoma virus is also a constitutively active cytoplasmic protein tyrosine kinase. The carboxy-terminal region of c-SRC is truncated in v-SRC, which results in deletion of tyrosine 527 and in constitutive activation of the v-SRC protein tyrosine kinase (25). A wide range of cellular substrates such as JAK and STAT proteins become phosphorylated in response to SRC (26, 27); v-SRC transforms fibroblasts and SRC kinase activity is enhanced in a variety of solid tumors (28).
Here we demonstrate that the BCR-ABL protein tyrosine kinase complements the EpoR and induces normal proliferation and differentiation of committed EpoR−/− fetal liver erythroid progenitors. A functional tyrosine kinase domain is essential for this rescue of erythropoiesis, but two BCR-ABL domains (residue Y177 and SH2 domain) that are essential for transformation of fibroblasts and/or bone marrow cells are dispensable. Finally, v-SRC, another constitutively active cytoplasmic protein tyrosine kinase unrelated to BCR-ABL, also induces normal erythroid development in EpoR−/− cells. These results indicate that terminal differentiation of erythroid cells requires generic signals provided by activated protein tyrosine kinases and proceeds without a specific set of signals unique to a cytokine receptor.
Methods
Cell Preparation and Retroviral Transduction.
Individual EpoR−/− fetal livers obtained at day 12.5 of embryogenesis were dissected in PBS containing 2% FCS, were disaggregated into single cell suspensions, were passed through a cell strainer (70 μm), and were washed two times in PBS with 2% FCS (1). A portion of the cells were diluted in 2% acetic acid to lyse mature erythrocytes and were counted. Retroviral supernatants were generated as follows: 10 μg of either MSCV-P210, MSCV-P210 kinase defective, MSCV-P210 Y177F, MSCV-P210 ΔSH2, MSCV-v-SRC-IRES-GFP or MSCV-EpoR-IRES-GFP DNA together with the PCL-Eco vector (29) were cotransfected (Invitrogen calcium phosphate kit) into the Phoenix packaging cell line (Garry Nolan, Stanford University), and the resulting viral supernatant was collected 48 hours later and used for infection of fetal liver cells. Fetal liver cells were resuspended in either viral supernatants at a multiplicity of infection of 5–10 or in the control Phoenix packaging line media and were incubated at 37°C for 3 hours in the presence of 4 μg/ml of polybrene, then were centrifuged at 2,000 rpm (Sorvall RT7, Sorvall) for 30 minutes. The viral titers were determined by infecting 3T3 cells using dilutions of viral supernatants. Western blot analysis determined the level of expression of retroviral-encoded proteins in Phoenix cells. Supernatants of equal viral titers were used in each experiment.
Erythroid-Colony-Forming Assays.
After retroviral transduction, day 12.5 EpoR−/− fetal liver cells were washed once in PBS, were resuspended in Iscove’s modified Dulbecco’s medium containing 2% FCS, and were plated in duplicate in cultures of 0.9% methylcellulose in Iscove’s modified Dulbecco’s medium containing 15% FBS, 1% BSA, 10 μg/ml bovine insulin, 200 μg/ml human transferrin, 10−4 M 2-mercaptoethanol, and 2 mM l-glutamine (MethoCult M3234, StemCell Technologies, Vancouver). The CFU-E assay was performed in these methylcellulose cultures containing 1% spleen conditioned media with or without Epo (3 units/ml; Amgen Biologicals). The BFU-E assay was performed in methylcellulose cultures containing either Epo alone (3 units/ml) or a combination of IL-3 (10 ng/ml), IL-6 (10 ng/ml), and SF (100 ng/ml) with or without Epo. The number of CFU-E colonies was determined after diaminobenzidine staining of hemoglobin and was counted after 2 days. BFU-E colonies of hemoglobinized red cells were counted after 8 days. Colonies were picked for cytospin, and their erythroblast morphology were determined after May–Grünwald Giemsa staining. Plates were coded and colonies were counted by a blinded observer.
Retroviral Constructs.
MSCVP210 was created by inserting a BCR-ABL cDNA encoding the 210-kDa form of BCR-ABL (10) into EcoRI-digested pMSCVpac (30); MSCVP210Y177F was created by ligating an EcoRI-XhoI fragment from P185BCR-ABL (Y177F) containing the Y177F Grb2 mutation (23) and an XhoI-EcoRI fragment from MSCVP210 containing the 3′ end of the P210BCR-ABL cDNA into EcoRI-digested pMSCVpac. MSCVP210K− was created by ligating an EcoRI-KpnI fragment fromMSCVP210 containing the 5′ end of the P210BCR-ABL cDNA and a KpnI-EcoRI fragment from P185BCR-ABL (Y813F) (Kinase-mutant) containing the Y813F Kinase mutation into EcoRI-digested pMSCVpac (31). The P210 ΔSH2 cDNA (32) was subcloned as an EcoRI fragment into the retroviral vector pMSCVpac. The EpoR c-DNA was amplified by PCR using 5′ (5′CGACGCGTCCTGAAGCTAGGGCTGCATCATGGAC3′) and 3′(5′CGACGTCGACGCGGCCGCCTGGAGTCCTAGGAGCAGGC3′) primers containing 5′MLU1 and 3′SalI, NotI restriction sites and ligated into the MLU-1, XhoI sites of the MSCV-IRES-GFP vector. v-SRC cDNA was PCR amplified from MMTV-v-SRC using 5′ HpaI (5′CGCGATGTTAACAGCCGACCACCATGGGGAGTAGC3′) and 3′XhoI (5′CGATCGCTCGAGGCTCGCGCACTACTCAGCGACC3′) ligated into the HpaI, XhoI sites of MSCV-IRES GFP vector. All c-DNAs amplified by PCR were subsequently sequenced.
Genomic PCR.
Individual BCR-ABL transduced BFU-E colonies plated in the presence of 15% serum only or serum supplemented with IL-3, IL-6, and SF were picked and genomic DNA was isolated [blood sample purification kit (Qiagen, Chatsworth, CA)] and was subjected to a PCR reaction using a 5′ primer from BCR (5′AGCATGGCCTTCAGGGTGCACAGCCGCAACGGCAA3′) and a 3′ primer from ABL (5′TCACTGGGTCCAGCGAGAAGGTTTTCCTTGGAGTT3′) (33).
Results
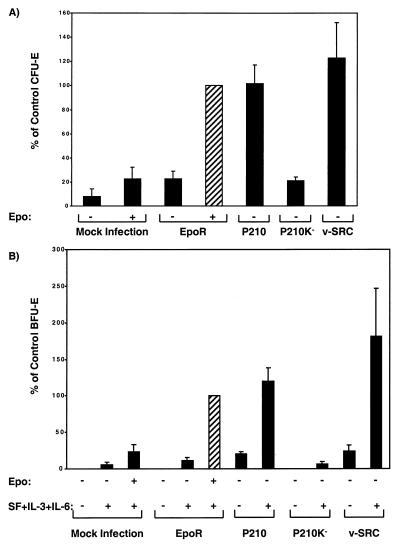
Using retroviral transduction, we introduced P210BCR-ABL into fetal liver progenitors cells obtained at day 12.5 of embryogenesis from EpoR−/− mice and observed rescue of erythroid CFU-E colonies (Fig. 1A). With viral supernatants of comparable high titers, BCR-ABL induced the formation of the same number of CFU-E colonies in the absence of Epo as in those infected with the EpoR retrovirus and supplemented with Epo (Fig. 1A). The CFU-E colonies generated by BCR-ABL were indistinguishable in size, morphology, and degree of hemoglobinization from CFU-E colonies generated by the EpoR and cultured in the presence of Epo (data not shown). The efficiency of retroviral infection of fetal liver cells is ≈10–20% (34, 35); given that wild-type fetal livers contain ≈2,000 CFU-Es per 105 cells at day 14 of embryogenesis, we conclude that virtually every CFU-E expressing a transduced EpoR or P210BCR-ABL protein differentiates into an erythroid colony (refs. 34 and 35; see Fig. 1). To examine the specificity of BCR-ABL protein tyrosine kinase in rescue of the erythroid phenotype, we expressed v-SRC, an unrelated cytoplasmic protein tyrosine kinase in EpoR−/− fetal liver cells. Surprisingly, v-SRC also supported erythroid differentiation of CFU-E progenitors (Fig. 1A). As with colonies generated by BCR-ABL, the v-SRC-induced erythroid colonies were normal in size, morphology, and degree of hemoglobinization (data not shown).
Figure 1.
Hematopoietic progenitor assays for BCR-ABL and v-SRC transduced EpoR−/− fetal liver cells. CFU-E (A) and BFU-E (B) colonies were generated in cultures of 105 EpoR−/− fetal liver cells infected with retroviral constructs encoding EpoR, BCR-ABL, and v-SRC. Total CFU-E and BFU-E were counted after 2 (A) and 8 days (B), respectively. Results are presented as percentage of EpoR rescued colonies in the optimum conditions (cultures containing Epo, IL-3, IL-6, and SF) shown by the hatched bars. Graphs are from duplicate cultures of 3 (v-SRC) to 10 (all other constructs) independent experiments. Under the optimum condition (cultures containing spleen conditioned media and Epo), the average of the absolute number of CFU-E colonies rescued by EpoR was 1,698 ± 426 (n = 4) per 106 cells plated. Similarly, the average of absolute number of BFU-E rescued by EpoR-infected cells under the optimum condition (Epo, IL-3, IL-6, and SF) was 68 ± 12 per 105 cells (n = 10).
For maximal proliferation and differentiation of BFU-E progenitors from wild-type or EpoR−/− fetal liver cells transduced by the EpoR retrovirus, Epo is required together with a mixture of growth factors including IL-3, IL-6, and SF (2, 36, 37). We confirmed this requirement for IL-3, IL-6, and SF, in addition to Epo (Fig. 1B). In the absence of the EpoR, addition of IL-3, IL-6, and SF induced only background levels of erythroid colonies (Fig. 1B, Bar 2), which contained nonhemoglobinized apoptotic erythroblasts (data not shown).
Infection of EpoR−/− fetal liver cells with either the P210BCR-ABL or v-SRC retrovirus fully supported differentiation of BFU-E progenitors and erythroid maturation in the presence of IL-3, IL-6, and SF (Fig. 1B). Importantly, a small but significant number of erythroid bursts were obtained from both P210BCR-ABL and v-SRC expressing EpoR−/− cells cultured in the absence of added IL-3, IL-6, and SF (Fig. 1B), suggesting that BCR-ABL and v-SRC partially complement these signaling pathways as well. Even in the absence of growth factor supplementation, all BCR-ABL and v-SRC generated bursts were comparable in size, morphology, and degree of hemoglobinization to BFU-E colonies generated by the EpoR and cultured in the presence of Epo and added growth factors (Fig. 2 A and B). Comparable results were obtained with wild-type fetal liver cells subjected to retroviral transduction of BCR-ABL (data not shown). Interestingly, Epo and BCR-ABL did not appear to have any synergistic effect on erythroid colony-formation of wild-type fetal liver cells, indicating that they stimulate overlapping signaling pathways.
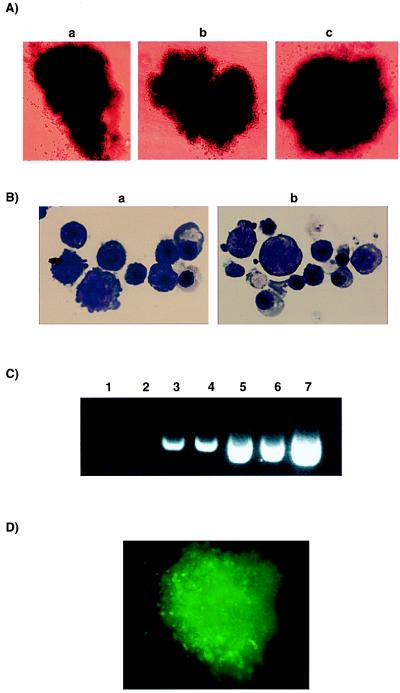
Figure 2.
BCR-ABL and v-SRC expression reconstitute the erythroid phenotype in EpoR−/− fetal liver cells. (A) Epo-independent BCR-ABL-generated bursts (BFU-E) cultured either in 15% serum alone (a) or in serum supplemented with IL-3, IL-6, and SF (b); Epo-dependent EpoR-generated colony cultured in Epo and IL3, IL-6, and SF is shown as control (c) (×100). (B) Cytospin preparations and May-Grünwald Giemsa staining of individual BCR-ABL-generated BFU-E-derived colonies generated in the absence (a) or presence of IL-3, IL-6, and SF (b) show erythroid cells at different stages of differentiation (×1,000). (C) Genomic PCR of BCR-ABL on individual BFU-E from EpoR (lanes 1 and 2)- or BCR-ABL (lanes 3–6)-derived colonies plated in the presence of 15% serum only (lanes 5 and 6), serum supplemented with IL-3, IL-6, and SF (lanes 3 and 4), or Epo and IL-3, IL-6, and SF (lanes 1 and 2). The PCR reaction was run on a 1.5% agarose gel and shows a band of 404 bp corresponding to the amplified breakpoint region of BCR-ABL. A positive control using a BCR-ABL containing plasmid MSCV-P210 is shown (lane 7). (D) GFP-positive v-SRC reconstituted BFU-E from EpoR−/− fetal liver cells (×100). Results are from one representative experiment.
Genomic PCR performed on individual Epo-independent erythroid colonies induced by BCR-ABL demonstrated that they contained the BCR-ABL gene (Fig. 2C). Variation between intensity of nonquantitative PCR bands was attributable to the variation of size of colonies picked. The bicistronic retroviral vector used to express v-SRC also expressed the green fluorescent protein (GFP). All erythroid colonies rescued by infection with the v-SRC retrovirus demonstrated green fluorescence, indicating that they expressed the MSCV-v-SRC-IRES-GFP construct (Fig. 2D).
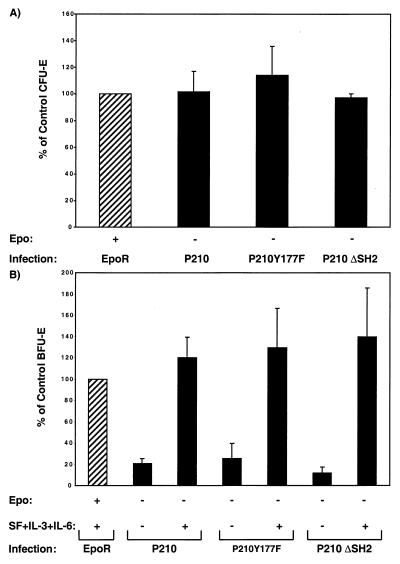
A kinase-defective mutant of P210BCR-ABL failed to induce any significant erythroid maturation (Fig. 1 A and B), indicating that a functional tyrosine kinase domain is essential for rescuing erythroid differentiation in EpoR−/− progenitors. In contrast, expression of a BCR-ABL containing a point mutation of the Y177 residue (Y177F) or a deletion of the SH2 domain efficiently rescued erythroid differentiation in EpoR−/− fetal liver cells (Fig. 3 A and B). The titers of these viruses were the same as those expressing the wild-type BCR-ABL, and these mutants rescued the erythroid phenotype in EpoR−/− fetal liver cells as well as native BCR-ABL. Genomic PCR confirmed the integration of BCR-ABL mutants in erythroid progenitors (data not shown). These results indicate that BCR-ABL rescue of erythroid development does not depend on activation of Ras via Grb-2-binding to tyrosine Y177 or on protein–protein interactions mediated by the ABL SH2 domain.
Figure 3.
BCR-ABL mutants rescue erythroid phenotype in EpoR−/− fetal liver cells. CFU-E (A) and BFU-E (B) colonies generated in cultures of 105 EpoR−/− fetal liver cells infected with retroviral constructs encoding BCR-ABL mutants (P210Y177F and P210 ΔSH2), wild-type P210BCR-ABL, or EpoR. Total CFU-E and BFU-E were counted after 2 (A) and 8 days (B), respectively. Results are presented as percentage of EpoR-rescued colonies in the optimum conditions shown by hatched bars (see legend of Fig. 1 A and B). Graphs are from duplicate cultures of at least four independent experiments.
Discussion
Recent work suggests that a major function of the EpoR is to prevent apoptosis of committed erythroid progenitors (38). Some of this function can likewise be provided by the cytoplasmic signaling domains of a variety of hematopoietic and nonhematopoietic receptors, including thrombopoietin, granulocyte colony stimulating factor, growth hormone, and prolactin receptors (3, 34, 35, 39). Here we showed that two nonreceptor tyrosine kinase oncoproteins, BCR-ABL and v-SRC, can complement for EpoR signaling and fully support normal erythroid development in EpoR-deficient fetal liver cells. These results demonstrate that activated cytoplasmic protein tyrosine kinases can fully replace EpoR function in terminal red blood cell development by mimicking critical signaling cascades.
Of the many signaling proteins activated by the EpoR, the importance of two, JAK2 and STAT5, have been validated by gene knock-out studies in mice (38, 40–42). JAK2−/− mice have a very similar phenotype to EpoR−/− mice and die around day 13–15 of embryogenesis from a severe anemia (40, 41). STAT5a−/−STAT5b−/− embryos, although viable, are severely anemic (38). Erythroid progenitors of these mice show high rates of apoptosis and a blunted response to Epo, findings explained by a crucial role for STAT5 in anti-apoptotic signaling by the EpoR. STAT5 mediates the immediate-early gene induction of bcl-X in erythroid cells through direct binding to the bcl-X gene (38). Presumably, cytokine receptors such as the prolactin receptor support EpoR−/− fetal liver erythropoiesis through activation of the JAK2/STAT5 pathway.
The BCR-ABL oncoprotein has been demonstrated to provide a potent anti-apoptotic signal to hematopoietic cells (43, 44). Both BCR-ABL and v-SRC have been shown to activate several JAK and STAT proteins (26, 27, 45–47). In addition, activation of STAT5 by BCR-ABL is essential for BCR-ABL leukemogenesis in vivo (48). However, because STAT5 mediates only some of the anti-apoptotic signals generated by the EpoR (38), BCR-ABL and v-SRC oncoproteins likely activate survival pathways in addition to STAT5 to rescue EpoR−/− erythroid progenitors. Whether BCR-ABL or v-SRC activate JAK2 or STAT5 in EpoR−/− fetal liver cells is unknown, but it is probable that the constitutive tyrosine kinase activity of these oncoproteins complements the tyrosine kinase function of JAK2 in addition to activating the STAT5 survival pathway.
BCR-ABL and v-SRC were as efficient as the EpoR in rescuing the development of erythroid colonies from EpoR−/− fetal livers. Importantly, BCR-ABL and v-SRC alone, in the absence of any added growth factors, supported normal proliferation and differentiation of a significant number of EpoR−/− bursts (Fig. 1B). This suggests that both of these oncoproteins partially complement signaling pathways induced by a number of cytokine receptors, including those for IL-3, IL-6, and SF, which are required to cooperate with the EpoR to allow full development of the erythroid lineage. Complementation of the BCR-ABL oncoprotein with these other cytokine receptors that act on early myeloid progenitors may play a role in the myeloproliferative phenotype observed in CML patients. We are currently testing whether BCR-ABL will rescue progenitors from the bone marrow of strains of mice deficient in myeloid cytokine receptors.
Although the EpoR prevents apoptosis and promotes differentiation of committed erythroid progenitors, expression of a constitutively active form of the EpoR (R129C) or the native EpoR itself in more primitive bone marrow cells has a very different effect: It induces proliferation of nonerythroid as well as immature erythroid progenitor cells but does not skew differentiation of nonerythroid lineages (49–51). Ectopic expression of the EpoR reveals its capacity to induce proliferation of multipotential progenitors; conversely, expression of transforming oncoproteins in committed erythroid progenitors, as in our experiments, demonstrates their ability to mimic signals that provide for terminal differentiation. Therefore, the cellular response to expression of the EpoR or activated cytoplasmic signaling proteins undoubtedly reflects the constellation of signaling proteins, transcription factors, and other proteins already expressed in the target cell.
Whether the BCR-ABL oncoprotein provides signals that induce cell differentiation has been controversial. In the human erythroleukemic cell line K562, derived from a CML patient, inhibition of BCR-ABL function results in erythroid differentiation (24). Although these results suggest that BCR-ABL expression in K562 cells inhibits erythroid differentiation, our results clearly demonstrate that expression of BCR-ABL in committed primary erythroid progenitors enables differentiation and production of fully hemoglobinized erythroid cells. In CML patients, there is a parallel increase in granulocytic and erythroid progenitors in peripheral blood (12), yet CML patients manifest granulocytosis, not erythrocytosis. The possibility of a block in differentiation of BCR-ABL-expressing erythroid progenitors appears unlikely, as our experiments show that Epo-independent colonies rescued by BCR-ABL are indistinguishable from their wild-type counterparts. Erythrocytosis may not be observed in CML patients because the red cell lineage remains dynamically regulated by Epo levels. Moreover, massive splenomegaly causes sequestration and destruction of red blood cells, which actually contributes to anemia in some patients (52).
Epo-independent growth and differentiation of erythroid progenitors in culture is a general characteristic of myeloproliferative diseases, disorders of hematopoietic stem cells characterized by a massive myeloid hyperplasia. In addition to CML, these include thrombocythemia, polycythemia vera, and myelofibrosis. The molecular basis for the other myeloproliferative disorders remains unknown, but it is likely that these diseases, like CML, share pathologic activation of cytokine signaling pathways. Our results provide a direct demonstration in primary cells that P210BCR-ABL can complement for cytokine receptor signaling. The inability of a kinase-defective BCR-ABL mutant to mediate rescue erythroid development and the ability of v-SRC to complement for EpoR function demonstrates that terminal differentiation of erythroid cells requires only “generic” signals provided by activated protein tyrosine kinases.
Acknowledgments
We thank Dr. Kevin Klucher for MSCVP210Pac, MSCV P210Y177F and MSCV P210K−, Dr. Philippe Leboulch (Massachusetts Institute of Technology) for the MSCV-IRES-GFP retroviral vector, Dr. Inder Verma (Salk Institute) for PCL-Eco vector, Dr. Ann Marie Pendergast (Duke University) for P185 Y177F c-DNA, and Drs. Roya Khosravi-Far and Richard Van Etten (Harvard Medical School) for the v-SRC and the P210 ΔSH2 cDNA, respectively. We are grateful to Drs. Bill Schiemann, Allen Sirotkin, Merav Socolovsky, and Stefan Constantinescu for helpful discussions and comments on the manuscript. We also thank Jen Cook-Chrysos for excellent technical assistance. This research was supported by National Cancer Institute Grant CA76418-01, the Burroughs-Wellcome Fund (to G.Q.D.), Grant HL 32262 from the National Institutes of Health, a grant from the Amgen Corporation to H.F.L., and in part by a National Institutes of Health Clinician Scientist Career Award to S.G.
Abbreviations
- Epo
erythropoietin
- EpoR
erythropoietin-receptor
- CML
chronic myeloid leukemia
- IL
interleukin
- SF
Steel factor
- CFU-E
colony-forming unit erythroid
- BFU-E
burst-forming unit erythroid
- GFP
green fluorescent protein
References
- 1.Wu H, Liu X, Jaenisch R, Lodish H F. Cell. 1995;83:59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- 2.Lin C S, Lim S K, D’Agati V, Costantini F. Genes Dev. 1996;10:154–164. doi: 10.1101/gad.10.2.154. [DOI] [PubMed] [Google Scholar]
- 3.Kieran M W, Perkins A C, Orkin S H, Zon L I. Proc Natl Acad Sci USA. 1996;93:9126–9131. doi: 10.1073/pnas.93.17.9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eaves A C, Eaves C J. Exp Hematol (Charlottesville, Va) 1979;5:65–75. [PubMed] [Google Scholar]
- 5.Rowley J D. Nature (London) 1973;243:290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 6.Shtivelman E, Lifshitz B, Gale R P, Canaani E. Nature (London) 1985;315:550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- 7.Konopka J B, Watanabe S M, Witte O N. Cell. 1984;37:1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- 8.Lugo T G, Pendergast A M, Muller A J, Witte O N. Science. 1990;247:1079–1082. doi: 10.1126/science.2408149. [DOI] [PubMed] [Google Scholar]
- 9.Gishizky M L, Witte O N. Science. 1992;256:836–839. doi: 10.1126/science.1375394. [DOI] [PubMed] [Google Scholar]
- 10.Daley G Q, Van Etten R A, Baltimore D. Science. 1990;247:824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- 11.Heisterkamp N, Jenster G, ten Hoeve J, Zovich D, Pattengale P K, Groffen J. Nature (London) 1990;344:251–253. doi: 10.1038/344251a0. [DOI] [PubMed] [Google Scholar]
- 12.Eaves C J, Eaves A C. Baillieres Clin Haematol. 1987;1:931–961. doi: 10.1016/s0950-3536(87)80033-1. [DOI] [PubMed] [Google Scholar]
- 13.Jiang X, Lopez A, Holyoake T, Eaves A, Eaves C. Proc Natl Acad Sci USA. 1999;96:12804–12809. doi: 10.1073/pnas.96.22.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Issaad C, Vainchenker W. Blood. 1994;84:3447–3456. [PubMed] [Google Scholar]
- 15.Constantinescu S N, Ghaffari S, Lodish H F. Trends Endocrinol. 1999;10:18–23. doi: 10.1016/s1043-2760(98)00101-5. [DOI] [PubMed] [Google Scholar]
- 16.Sawyers C L. Baillieres Clin Haematol. 1997;10:223–231. doi: 10.1016/s0950-3536(97)80004-2. [DOI] [PubMed] [Google Scholar]
- 17.McWhirter J R, Wang J Y. Mol Cell Biol. 1991;11:1553–1565. doi: 10.1128/mcb.11.3.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McWhirter J R, Galasso D L, Wang J Y. Mol Cell Biol. 1993;13:7587–7595. doi: 10.1128/mcb.13.12.7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayer B J, Baltimore D. Mol Cell Biol. 1994;14:2883–2894. doi: 10.1128/mcb.14.5.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skorski T, Nieborowska-Skorska M, Wlodarski P, Wasik M, Trotta R, Kanakaraj P, Salomoni P, Antonyak M, Martinez R, Majewski M, et al. Blood. 1998;91:406–418. [PubMed] [Google Scholar]
- 21.Mayer B J, Jackson P K, Van Etten R A, Baltimore D. Mol Cell Biol. 1992;12:609–618. doi: 10.1128/mcb.12.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goga A, McLaughlin J, Afar D E, Saffran D C, Witte O N. Cell. 1995;82:981–988. doi: 10.1016/0092-8674(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 23.Pendergast A M, Quilliam L A, Cripe L D, Bassing C H, Dai Z, Li N, Batzer A, Rabun K M, Der C J, Schlessinger J, et al. Cell. 1993;75:175–185. [PubMed] [Google Scholar]
- 24.Gishizky M L, Cortez D, Pendergast A M. Proc Natl Acad Sci USA. 1995;92:10889–10893. doi: 10.1073/pnas.92.24.10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartzberg P L. Oncogene. 1998;17:1463–1468. doi: 10.1038/sj.onc.1202176. [DOI] [PubMed] [Google Scholar]
- 26.Campbell G S, Yu C L, Jove R, Carter-Su C. J Biol Chem. 1997;272:2591–2594. doi: 10.1074/jbc.272.5.2591. [DOI] [PubMed] [Google Scholar]
- 27.Chaturvedi P, Sharma S, Reddy E P. Mol Cell Biol. 1997;17:3295–3304. doi: 10.1128/mcb.17.6.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Messerschmitt A S, Dunant N, Ballmer-Hofer K. Virology. 1997;227:271–280. doi: 10.1006/viro.1996.8316. [DOI] [PubMed] [Google Scholar]
- 29.Naviaux R K, Costanzi E, Haas M, Verma I M. J Virol. 1996;70:5701–5705. doi: 10.1128/jvi.70.8.5701-5705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hawley T S, Sabourin L A, Hawley R G. Plasmid. 1989;22:120–131. doi: 10.1016/0147-619x(89)90021-8. [DOI] [PubMed] [Google Scholar]
- 31.Afar D E, Goga A, McLaughlin J, Witte O N, Sawyers C L. Science. 1994;264:424–426. doi: 10.1126/science.8153630. [DOI] [PubMed] [Google Scholar]
- 32.Ilaria R L, Jr, Van Etten R A. Blood. 1995;86:3897–3904. [PubMed] [Google Scholar]
- 33.Roth M S, Antin J H, Bingham E L, Ginsburg D. Blood. 1989;74:882–885. [PubMed] [Google Scholar]
- 34.Socolovsky M, Dusanter-Fourt I, Lodish H F. J Biol Chem. 1997;272:14009–14012. doi: 10.1074/jbc.272.22.14009. [DOI] [PubMed] [Google Scholar]
- 35.Socolovsky M, Fallon A E, Lodish H F. Blood. 1998;92:1491–1496. [PubMed] [Google Scholar]
- 36.Lansdorp P M, Dragowska W. J Exp Med. 1992;175:1501–1509. doi: 10.1084/jem.175.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papayannopoulou T, Brice M, Blau C A. Blood. 1993;81:299–310. [PubMed] [Google Scholar]
- 38.Socolovsky M, Fallon A E, Wang S, Brugnara C, Lodish H F. Cell. 1999;98:181–191. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 39.Goldsmith M A, Mikami A, You Y, Liu K D, Thomas L, Pharr P, Longmore G D. Proc Natl Acad Sci USA. 1998;95:7006–7011. doi: 10.1073/pnas.95.12.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neubauer H, Cumano A, Muller M, Wu H, Huffstadt U, Pfeffer K. Cell. 1998;93:397–409. doi: 10.1016/s0092-8674(00)81168-x. [DOI] [PubMed] [Google Scholar]
- 41.Parganas E, Wang D, Stravopodis D, Topham D J, Marine J C, Teglund S, Vanin E F, Bodner S, Colamonici O R, van Deursen J M, et al. Cell. 1998;93:385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 42.Teglund S, McKay C, Schuetz E, van Deursen J M, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle J N. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 43.Cortez D, Kadlec L, Pendergast A M. Mol Cell Biol. 1995;15:5531–5541. doi: 10.1128/mcb.15.10.5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kabarowski J H, Allen P B, Wiedemann L M. EMBO J. 1994;13:5887–5895. doi: 10.1002/j.1460-2075.1994.tb06934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carlesso N, Frank D A, Griffin J D. J Exp Med. 1996;183:811–820. doi: 10.1084/jem.183.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shuai K, Halpern J, ten Hoeve J, Rao X, Sawyers C L. Oncogene. 1996;13:247–254. [PubMed] [Google Scholar]
- 47.Ilaria R L, Jr, Van Etten R A. J Biol Chem. 1996;271:31704–31710. doi: 10.1074/jbc.271.49.31704. [DOI] [PubMed] [Google Scholar]
- 48.Nieborowska-Skorska M, Wasik M A, Slupianek A, Salomoni P, Kitamura T, Calabretta B, Skorski T. J Exp Med. 1999;189:1229–1242. doi: 10.1084/jem.189.8.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Longmore G D, Lodish H F. Cell. 1991;67:1089–1102. doi: 10.1016/0092-8674(91)90286-8. [DOI] [PubMed] [Google Scholar]
- 50.Pharr P N, Hankins D, Hofbauer A, Lodish H F, Longmore G D. Proc Natl Acad Sci USA. 1993;90:938–942. doi: 10.1073/pnas.90.3.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dubart A, Feger F, Lacout C, Goncalves F, Vainchenker W, Dumenil D. Mol Cell Biol. 1994;14:4834–4842. doi: 10.1128/mcb.14.7.4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mentzer S J, Osteen R T, Starnes H F, Moloney W C, Rosenthal D, Canellos G, Wilson R E. Ann Surg. 1987;205:13–17. doi: 10.1097/00000658-198701000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]