Abstract
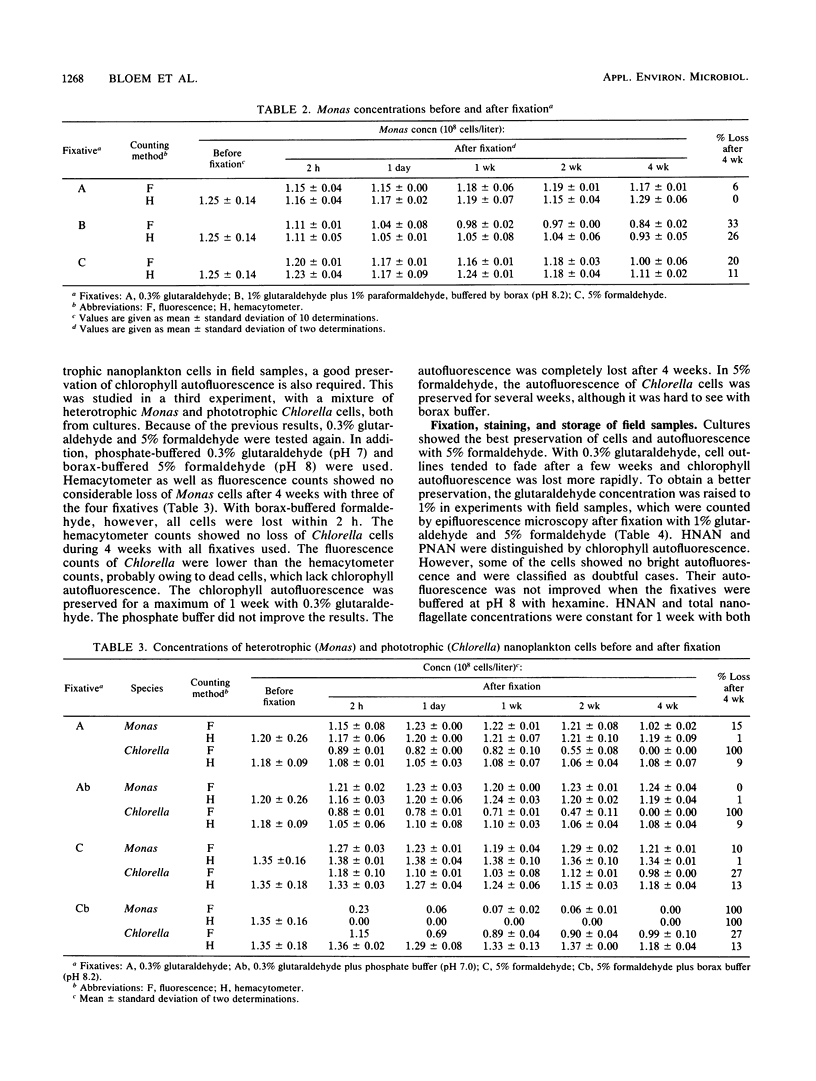
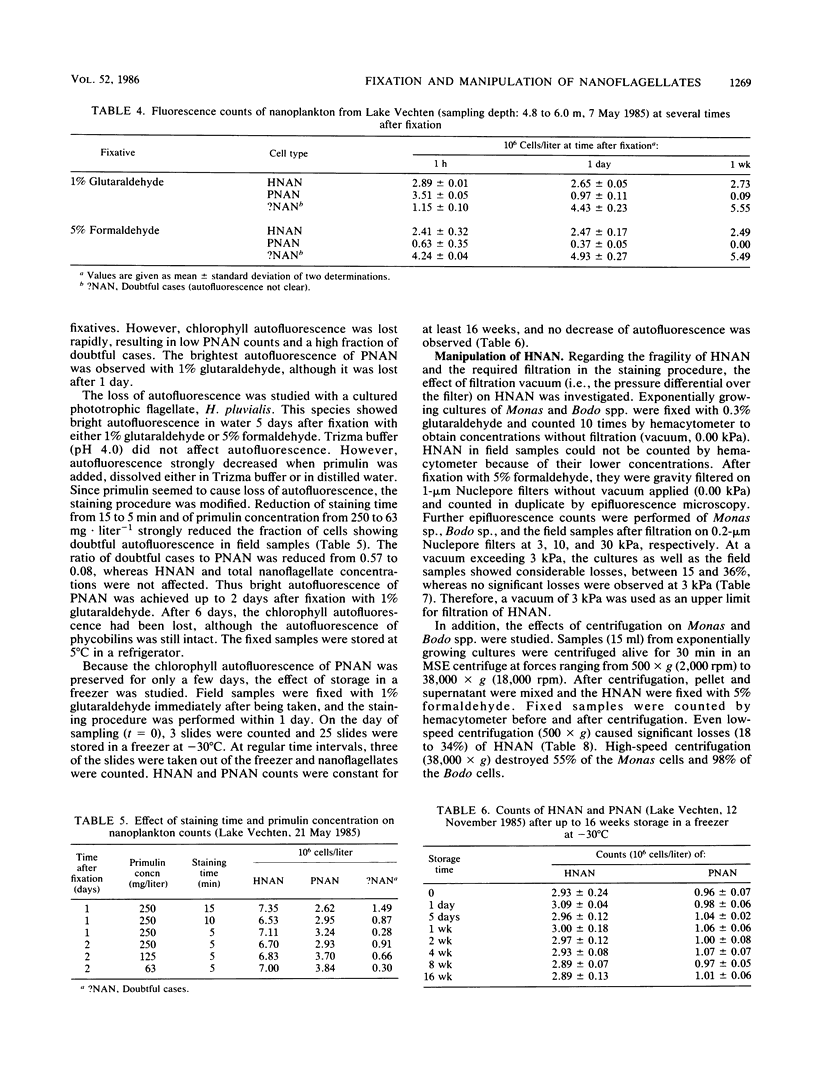
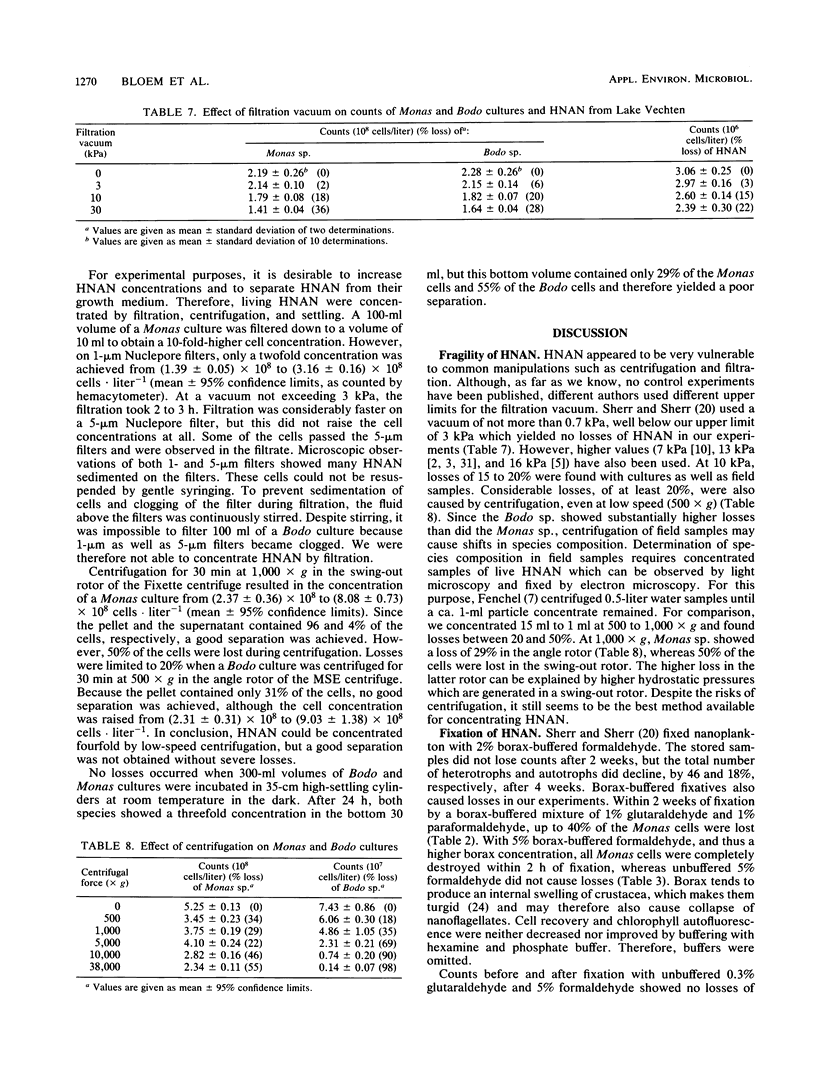
Quantitative effects of several fixatives on heterotrophic nanoflagellates (HNAN) and phototrophic nanoflagellates (PNAN) were investigated by hemacytometer and epifluorescence counting techniques. Counts of Monas sp. cultures before and after fixation with unbuffered 0.3% glutaraldehyde and 5% formaldehyde showed no loss of cells during fixation, and cell concentrations remained constant for several weeks after fixation. Buffering of fixatives with borax caused severe losses, up to 100% within 2 h. Field samples from Lake Vechten showed no decline of HNAN and total nanoflagellate concentrations for at least 1 week after fixation with 5% formaldehyde and with 1% glutaraldehyde. With 1% glutaraldehyde, the chlorophyll autofluorescence of PNAN was much brighter than with 5% formaldehyde, although it was lost after a few days and thus limited the storage time of samples. However, when primulin-stained slides were prepared soon after fixation and stored at −30°C, the loss of autofluorescence was prevented and PNAN and HNAN concentrations were stable for at least 16 weeks. Effects of filtration and centrifugation on HNAN were also studied. Filtration vacuum could not exceed 3 kPa since 10 kPa already caused losses of 15 to 20%. Similar losses were caused by centrifugation, even at low speed (500 × g).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caron D. A. Technique for enumeration of heterotrophic and phototrophic nanoplankton, using epifluorescence microscopy, and comparison with other procedures. Appl Environ Microbiol. 1983 Aug;46(2):491–498. doi: 10.1128/aem.46.2.491-498.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehority B. A. Evaluation of subsampling and fixation procedures used for counting rumen protozoa. Appl Environ Microbiol. 1984 Jul;48(1):182–185. doi: 10.1128/aem.48.1.182-185.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomroy A. J. Direct counting of bacteria preserved with lugol iodine solution. Appl Environ Microbiol. 1984 May;47(5):1191–1192. doi: 10.1128/aem.47.5.1191-1192.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr E. B., Sherr B. F. Double-staining epifluorescence technique to assess frequency of dividing cells and bacteriovory in natural populations of heterotrophic microprotozoa. Appl Environ Microbiol. 1983 Dec;46(6):1388–1393. doi: 10.1128/aem.46.6.1388-1393.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


