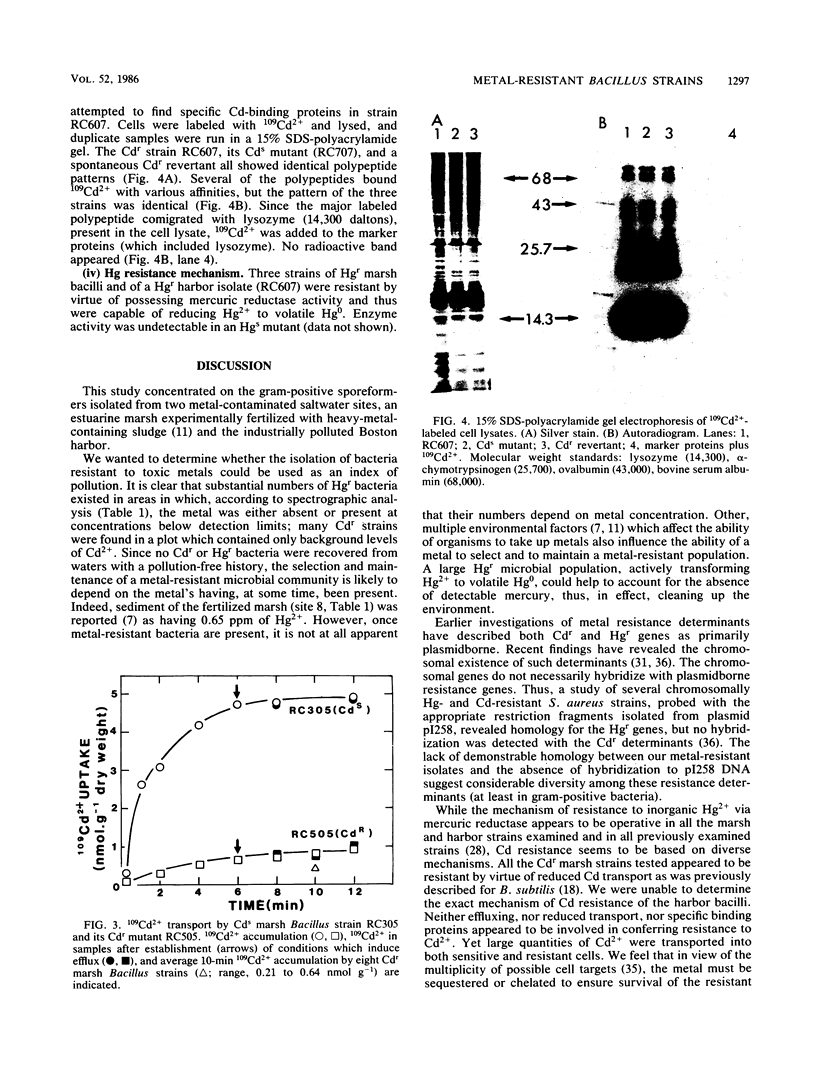
Abstract
Bacteria resistant to cadmium or mercury or both were isolated from the Great Sippewissett Marsh (Cape Cod, Mass.) and from Boston Harbor. Many of these metal-resistant isolates were gram-positive aerobic sporeformers, although not necessarily isolated as spores. Although several of the isolated strains bore plasmids, cadmium and mercury resistances appeared to be, for the most part, chromosomally encoded. DNA sequence homology of the gram-positive cadmium- and mercury-resistant isolates was not demonstrable with metal resistance genes from plasmids of either gram-positive (pI258) or gram-negative (pDB7) origin. Cadmium resistance of all the marsh isolates tested resulted from reduced Cd2+ transport. On the other hand, three cadmium-resistant harbor isolates displayed considerable influx but no efflux of Cd2+. Hg-resistant strains detoxified mercury by transforming Hg2+ to volatile Hg0 via mercuric reductase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald F. S., Duong M. N. Manganese acquisition by Lactobacillus plantarum. J Bacteriol. 1984 Apr;158(1):1–8. doi: 10.1128/jb.158.1.1-8.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkay T., Fouts D. L., Olson B. H. Preparation of a DNA gene probe for detection of mercury resistance genes in gram-negative bacterial communities. Appl Environ Microbiol. 1985 Mar;49(3):686–692. doi: 10.1128/aem.49.3.686-692.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrineau P., Gilbert P., Jackson W. J., Jones C. S., Summers A. O., Wisdom S. The DNA sequence of the mercury resistance operon of the IncFII plasmid NR1. J Mol Appl Genet. 1984;2(6):601–619. [PubMed] [Google Scholar]
- Bernhard K., Schrempf H., Goebel W. Bacteriocin and antibiotic resistance plasmids in Bacillus cereus and Bacillus subtilis. J Bacteriol. 1978 Feb;133(2):897–903. doi: 10.1128/jb.133.2.897-903.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bott K. F., Wilson G. A. Development of competence in the Bacillus subtilis transformation system. J Bacteriol. 1967 Sep;94(3):562–570. doi: 10.1128/jb.94.3.562-570.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Ehrlich S. D. Replication and expression of plasmids from Staphylococcus aureus in Bacillus subtilis. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1680–1682. doi: 10.1073/pnas.74.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J. Plasmid-determined resistance to antimicrobial drugs and toxic metal ions in bacteria. Microbiol Rev. 1983 Sep;47(3):361–409. doi: 10.1128/mr.47.3.361-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham D. P., Sadler P. J., Scawen M. D. Cadmium-Resistant Pseudomonas putida Synthesizes Novel Cadmium Proteins. Science. 1984 Sep 7;225(4666):1043–1046. doi: 10.1126/science.225.4666.1043. [DOI] [PubMed] [Google Scholar]
- Izaki K. Enzymatic reduction of mercurous and mercuric ions in Bacillus cereus. Can J Microbiol. 1981 Feb;27(2):192–197. doi: 10.1139/m81-030. [DOI] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazaeli M. B., Mitra R. S. Cadmium-binding component in Escherichia coli during accommodation to low levels of this ion. Appl Environ Microbiol. 1981 Jan;41(1):46–50. doi: 10.1128/aem.41.1.46-50.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laddaga R. A., Bessen R., Silver S. Cadmium-resistant mutant of Bacillus subtilis 168 with reduced cadmium transport. J Bacteriol. 1985 Jun;162(3):1106–1110. doi: 10.1128/jb.162.3.1106-1110.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler I., Halvorson H. O. Transformation of Escherichia coli and Bacillus subtilis with a hybrid plasmid molecule. J Bacteriol. 1977 Jul;131(1):374–377. doi: 10.1128/jb.131.1.374-377.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEntee J. D., Woodrow J. R., Quirk A. V. Investigation of cadmium resistance in an Alcaligenes sp. Appl Environ Microbiol. 1986 Mar;51(3):515–520. doi: 10.1128/aem.51.3.515-520.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. D., Silver S. Cadmium and manganese transport in Staphylococcus aureus membrane vesicles. J Bacteriol. 1982 May;150(2):973–976. doi: 10.1128/jb.150.2.973-976.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robinson J. B., Tuovinen O. H. Mechanisms of microbial resistance and detoxification of mercury and organomercury compounds: physiological, biochemical, and genetic analyses. Microbiol Rev. 1984 Jun;48(2):95–124. doi: 10.1128/mr.48.2.95-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Sakaguchi K. Construction of a recombinant plasmid composed of B. subtilis leucine genes and a B. subtilis (natto) plasmid: its use as cloning vehicle in B. subtilis 168. Mol Gen Genet. 1978 Oct 24;165(3):269–276. doi: 10.1007/BF00332526. [DOI] [PubMed] [Google Scholar]
- Thorne C. B., Stull H. B. Factors affecting transformation of Bacillus licheniformis. J Bacteriol. 1966 Mar;91(3):1012–1020. doi: 10.1128/jb.91.3.1012-1020.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynecka Z., Gos Z., Zajac J. Energy-dependent efflux of cadmium coded by a plasmid resistance determinant in Staphylococcus aureus. J Bacteriol. 1981 Aug;147(2):313–319. doi: 10.1128/jb.147.2.313-319.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Ulmer D. D. Biochemical effects of mercury, cadmium, and lead. Annu Rev Biochem. 1972;41(10):91–128. doi: 10.1146/annurev.bi.41.070172.000515. [DOI] [PubMed] [Google Scholar]
- Witte W., Green L., Misra T. K., Silver S. Resistance to mercury and to cadmium in chromosomally resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Apr;29(4):663–669. doi: 10.1128/aac.29.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]