Abstract
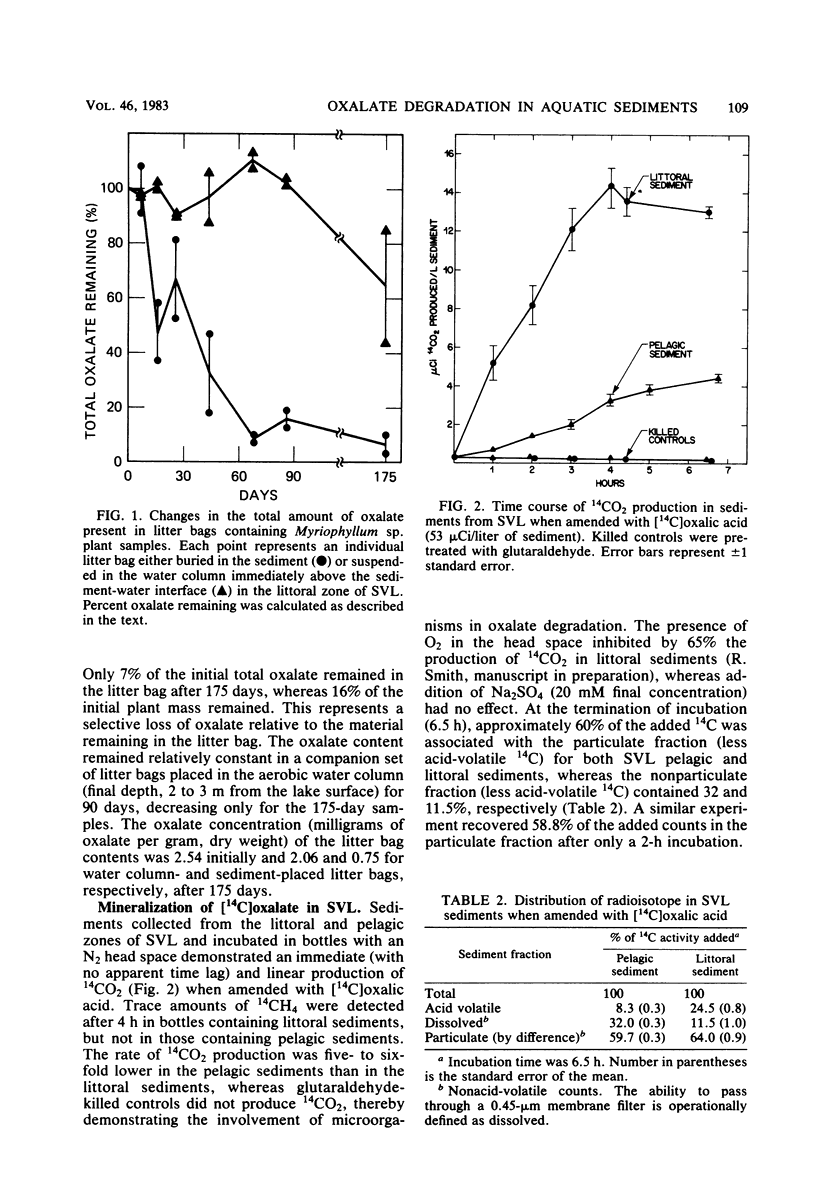
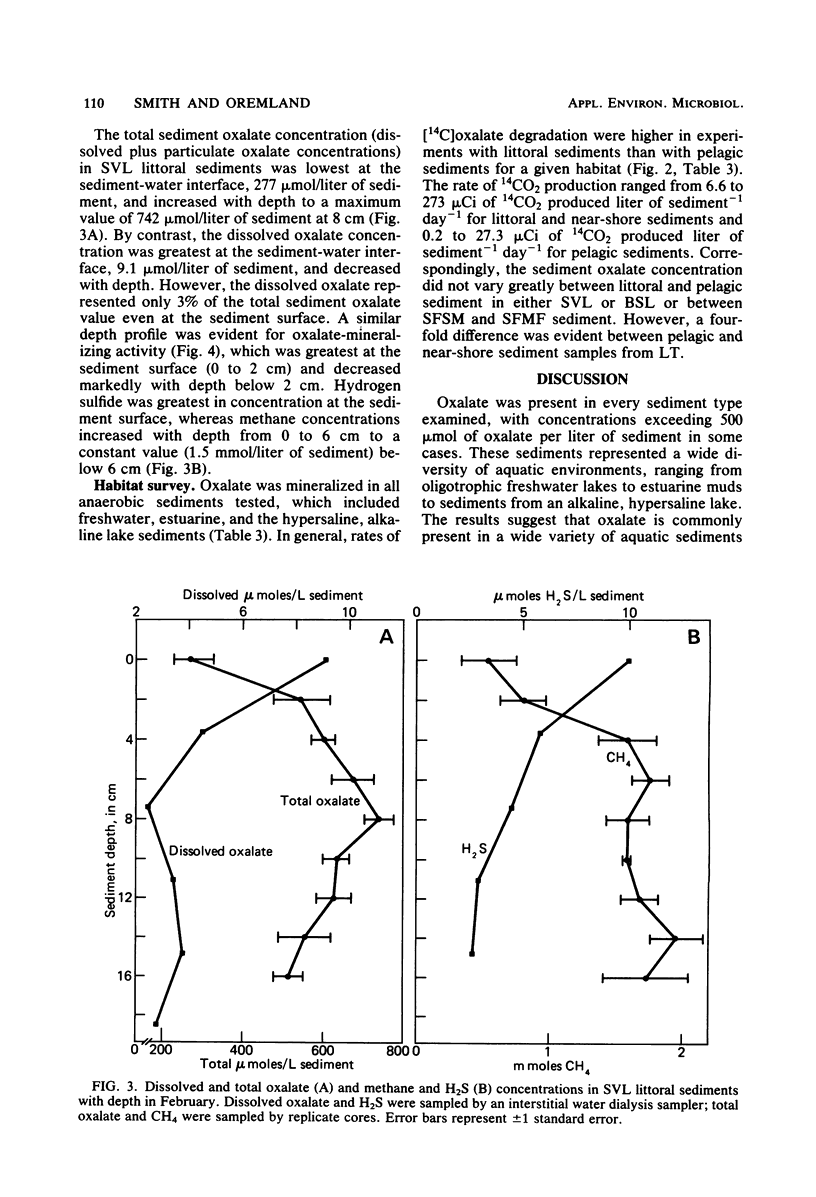
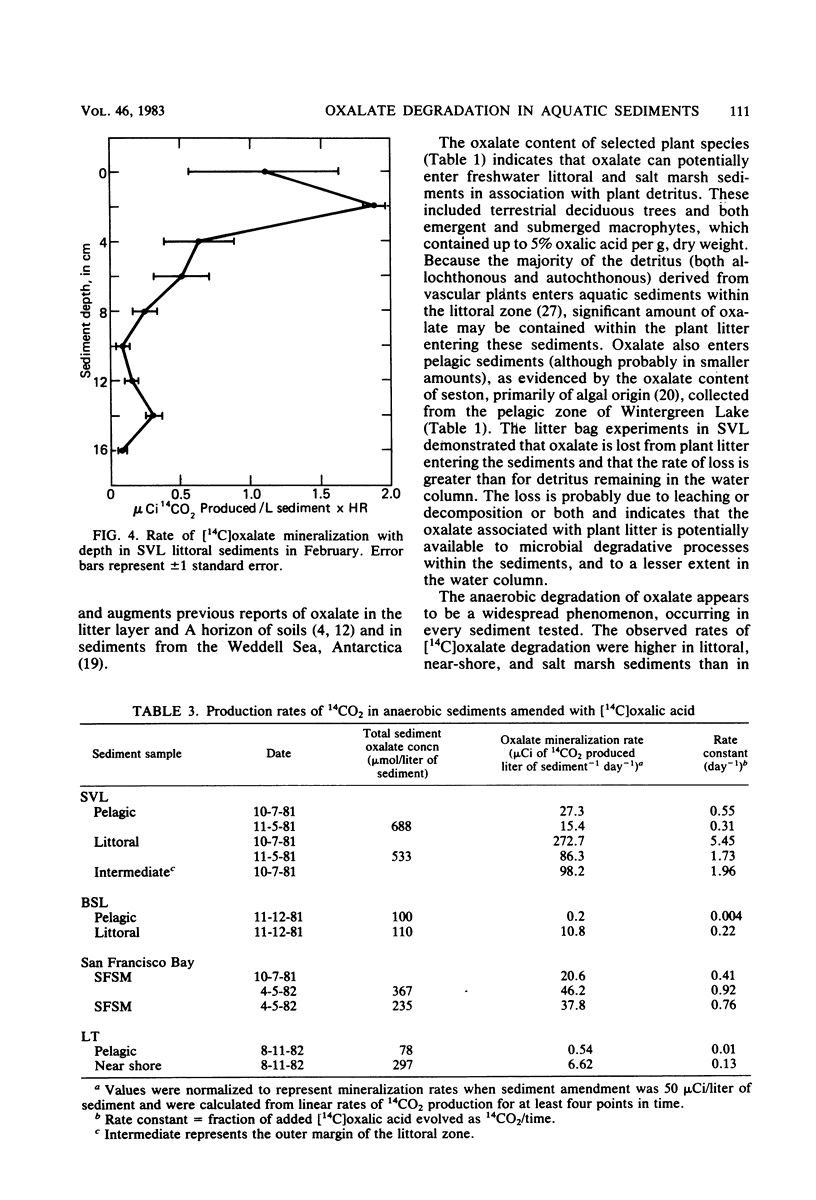
Significant concentrations of oxalate (dissolved plus particulate) were present in sediments taken from a diversity of aquatic environments, ranging from 0.1 to 0.7 mmol/liter of sediment. These included pelagic and littoral sediments from two freshwater lakes (Searsville Lake, Calif., and Lake Tahoe, Calif.), a hypersaline, meromictic, alkaline lake (Big Soda Lake, Nev.), and a South San Francisco Bay mud flat and salt marsh. The oxalate concentration of several plant species which are potential detrital inputs to these aquatic sediments ranged from 0.1 to 5.0% (wt/wt). In experiments with litter bags, the oxalate content of Myriophyllum sp. samples buried in freshwater littoral sediments decreased to 7% of the original value in 175 days. This suggests that plant detritus is a potential source of the oxalate within these sediments. [14C]oxalic acid was anaerobically degraded to 14CO2 in all sediment types tested, with higher rates evident in littoral sediments than in the pelagic sediments of the lakes studied. The turnover time of the added [14C]oxalate was less than 1 day in Searsville Lake littoral sediments. The total sediment oxalate concentration did not vary significantly between littoral and pelagic sediments and therefore did not appear to be controlling the rate of oxalate degradation. However, depth profiles of [14C]oxalate mineralization and dissolved oxalate concentration were closely correlated in freshwater littoral sediments; both were greatest in the surface sediments (0 to 5 cm) and decreased with depth. The dissolved oxalate concentration (9.1 μmol/liter of sediment) was only 3% of the total extractable oxalate (277 μmol/liter of sediment) at the sediment surface. These results suggest that anaerobic oxalate degradation is a widespread phenomenon in aquatic sediments and may be limited by the dissolved oxalate concentration within these sediments.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison M. J., Cook H. M. Oxalate degradation by microbes of the large bowel of herbivores: the effect of dietary oxalate. Science. 1981 May 8;212(4495):675–676. doi: 10.1126/science.7221555. [DOI] [PubMed] [Google Scholar]
- Allison M. J., Littledike E. T., James L. F. Changes in ruminal oxalate degradation rates associated with adaptation to oxalate ingestion. J Anim Sci. 1977 Nov;45(5):1173–1179. doi: 10.2527/jas1977.4551173x. [DOI] [PubMed] [Google Scholar]
- Culbertson C. W., Zehnder A. J., Oremland R. S. Anaerobic oxidation of acetylene by estuarine sediments and enrichment cultures. Appl Environ Microbiol. 1981 Feb;41(2):396–403. doi: 10.1128/aem.41.2.396-403.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson K. A., Allison M. J., Hartman P. A. Characteristics of anaerobic oxalate-degrading enrichment cultures from the rumen. Appl Environ Microbiol. 1980 Oct;40(4):840–846. doi: 10.1128/aem.40.4.840-846.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson K. A., Allison M. J., Hartman P. A. Isolation and some characteristics of anaerobic oxalate-degrading bacteria from the rumen. Appl Environ Microbiol. 1980 Oct;40(4):833–839. doi: 10.1128/aem.40.4.833-839.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich G. G., Goerlitz D. F., Bourell J. H., Eisen G. V., Godsy E. M. Liquid chromatographic procedure for fermentation product analysis in the identification of anaerobic bacteria. Appl Environ Microbiol. 1981 Nov;42(5):878–885. doi: 10.1128/aem.42.5.878-885.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graustein W. C., Cromack K., Jr, Sollins P. Calcium oxalate: occurrence in soils and effect on nutrient and geochemical cycles. Science. 1977 Dec 23;198(4323):1252–1254. doi: 10.1126/science.198.4323.1252. [DOI] [PubMed] [Google Scholar]
- JAKOBY W. B., BHAT J. V. Microbial metabolism of oxalic acid. Bacteriol Rev. 1958 Jun;22(2):75–80. doi: 10.1128/br.22.2.75-80.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James L. F., Street J. C., Butcher J. E. In vitro degradation of oxalate and of cellulose by rumen ingesta from sheep fed Halogeton glomeratus. J Anim Sci. 1967 Nov;26(6):1438–1444. doi: 10.2527/jas1967.2661438x. [DOI] [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Intermediary metabolism of organic matter in the sediments of a eutrophic lake. Appl Environ Microbiol. 1982 Mar;43(3):552–560. doi: 10.1128/aem.43.3.552-560.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstam H. A. Weddellite in a marine gastropod and in antarctic sediments. Science. 1968 Dec 6;162(3858):1129–1130. doi: 10.1126/science.162.3858.1129. [DOI] [PubMed] [Google Scholar]
- Oremland R. S., Marsh L., Desmarais D. J. Methanogenesis in big soda lake, nevada: an alkaline, moderately hypersaline desert lake. Appl Environ Microbiol. 1982 Feb;43(2):462–468. doi: 10.1128/aem.43.2.462-468.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Slack C. R. Simple methods for routine screening and quantitative estimation of oxalate content of tropical grasses. J Sci Food Agric. 1973 Jul;24(7):803–811. doi: 10.1002/jsfa.2740240708. [DOI] [PubMed] [Google Scholar]
- Smith R. L., Klug M. J. Electron donors utilized by sulfate-reducing bacteria in eutrophic lake sediments. Appl Environ Microbiol. 1981 Jul;42(1):116–121. doi: 10.1128/aem.42.1.116-121.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Klug M. J. Reduction of sulfur compounds in the sediments of a eutrophic lake basin. Appl Environ Microbiol. 1981 May;41(5):1230–1237. doi: 10.1128/aem.41.5.1230-1237.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]



