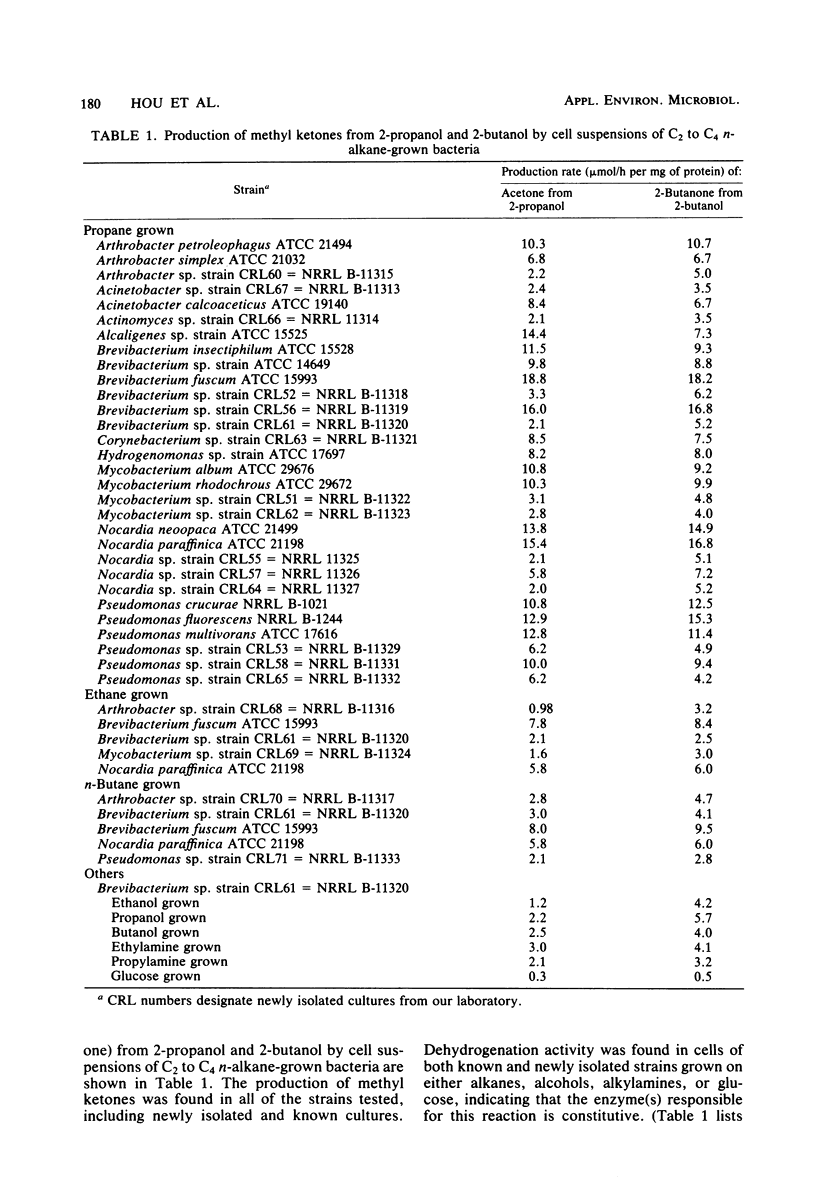
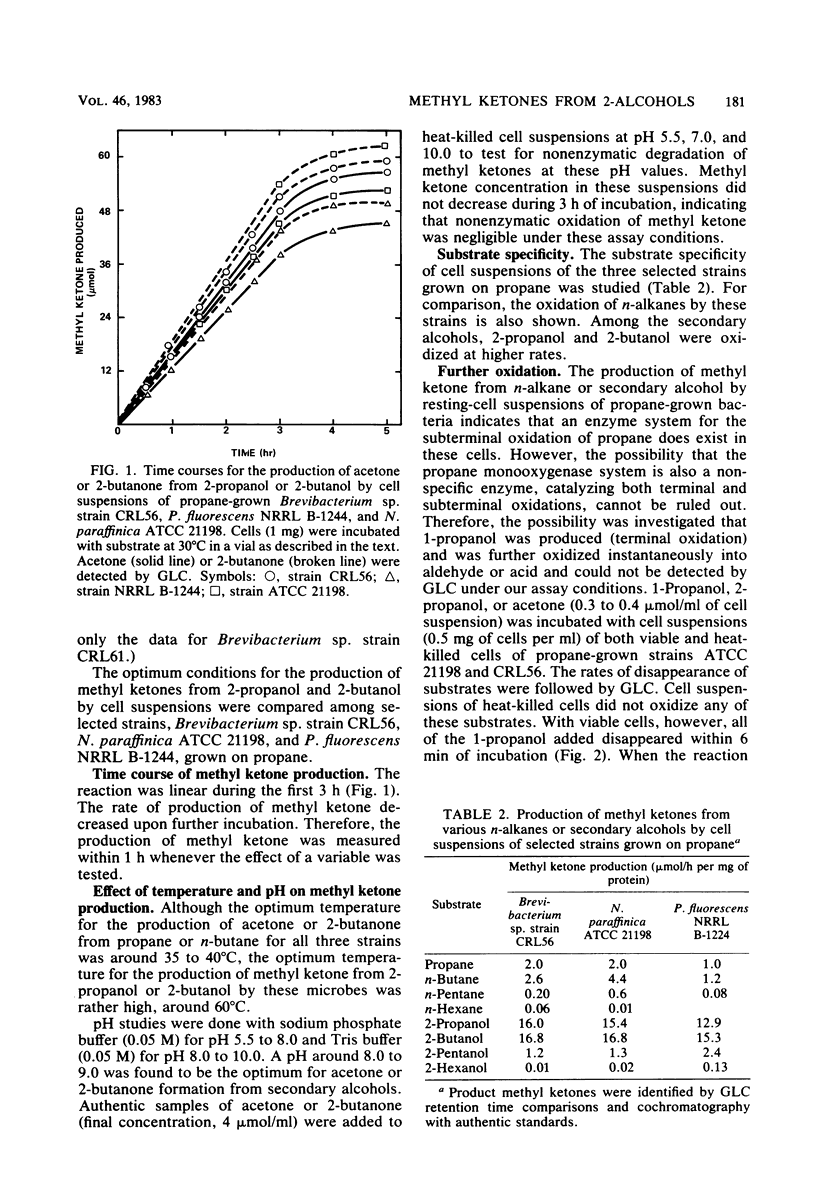
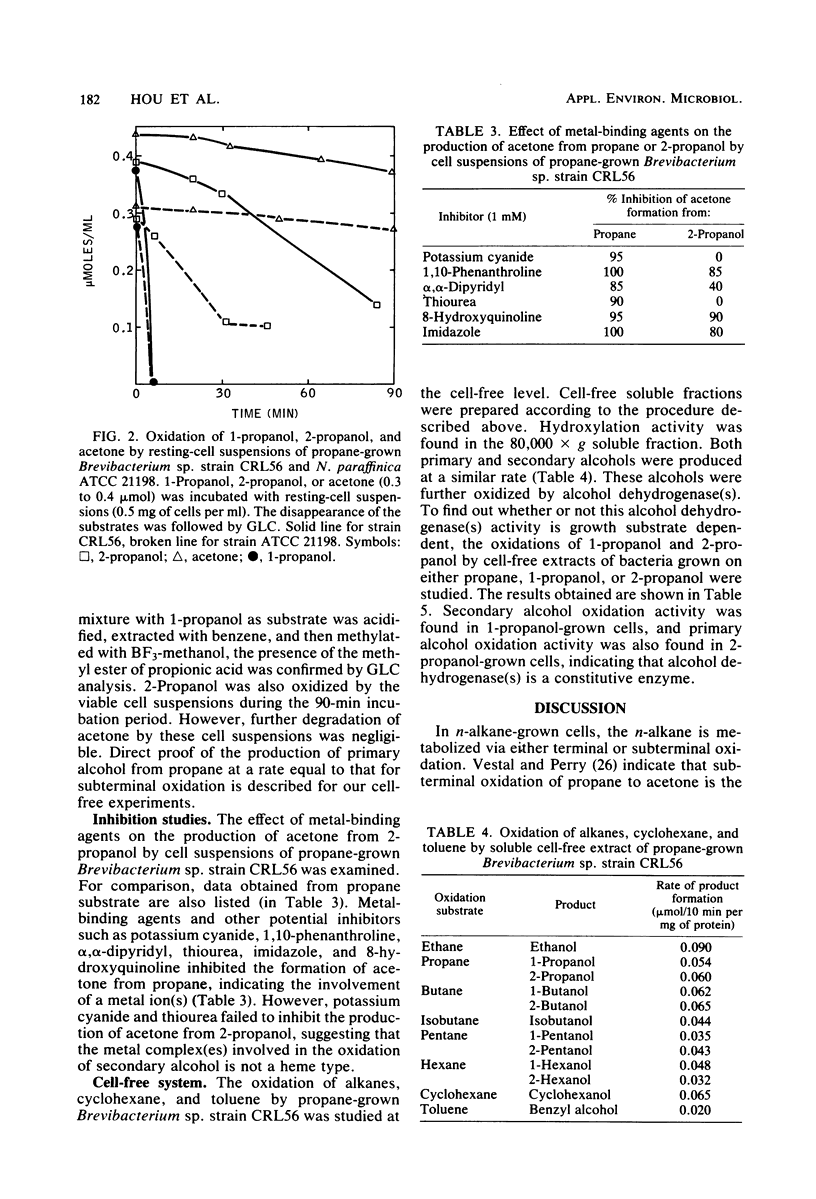
Abstract
Nineteen new C2 to C4n-alkane-grown cultures were isolated from lake water from Warinanco Park, Linden, N.J., and from lake and soil samples from Bayway Refinery, Linden, N.J. Fifteen known liquid alkane-utilizing cultures were also found to be able to grow on C2 to C4n-alkanes. Cell suspensions of these C2 to C4n-alkane-grown bacteria oxidized 2-alcohols (2-propanol, 2-butanol, 2-pentanol, and 2-hexanol) to their corresponding methyl ketones. The product methyl ketones accumulated extracellularly. Cells grown on 1-propanol or 2-propanol oxidized both primary and secondary alcohols. In addition, the activity for production of methyl ketones from secondary alcohols was found in cells grown on either alkanes, alcohols, or alkylamines, indicating that the enzyme(s) responsible for this reaction is constitutive. The optimum conditions for in vivo methyl ketone formation from secondary alcohols were compared among selected strains: Brevibacterium sp. strain CRL56, Nocardia paraffinica ATCC 21198, and Pseudomonas fluorescens NRRL B-1244. The rates for the oxidation of secondary alcohols were linear for the first 3 h of incubation. Among secondary alcohols, 2-propanol and 2-butanol were oxidized at the highest rate. A pH around 8.0 to 9.0 was found to be the optimum for acetone or 2-butanone formation from 2-alcohols. The temperature optimum for the production of acetone or 2-butanone from 2-propanol or 2-butanol was rather high at 60°C, indicating that the enzyme involved in the reaction is relatively thermally stable. Metal-chelating agents inhibit the production of methyl ketones, suggesting the involvement of a metal(s) in the oxidation of secondary alcohols. Secondary alcohol dehydrogenase activity was found in the cell-free soluble fraction; this activity requires a cofactor, specifically NAD. Propane monooxygenase activity was also found in the cell-free soluble fraction. It is a nonspecific enzyme catalyzing both terminal and subterminal oxidation of n-alkanes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott B. J., Hou C. T. Oxidation of 1-alkenes to 1,2-epoxyalkanes by Pseudomonas oleovorans. Appl Microbiol. 1973 Jul;26(1):86–91. doi: 10.1128/am.26.1.86-91.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett C. H., Dodgson K. S., White G. F., Payne W. J. Preliminary observations on alcohol dehydrogenases in Comamonas terrigena that exhibit stereospecificity towards secondary alcohols. Biochem J. 1980 Jun 1;187(3):703–709. doi: 10.1042/bj1870703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C. T., Patel R. N., Laski A. I., Marczak I., Barnabe N. Microbial oxidation of gaseous hydrocarbons: production of alcohols and methyl ketones from their corresponding n-alkanes by methylotrophic bacteria. Can J Microbiol. 1981 Jan;27(1):107–115. doi: 10.1139/m81-017. [DOI] [PubMed] [Google Scholar]
- Hou C. T., Patel R. N., Laskin A. I., Barist I., Barnabe N. Thermostable NAD-linked secondary alcohol dehydrogenase from propane-grown Pseudomonas fluorescens NRRL B-1244. Appl Environ Microbiol. 1983 Jul;46(1):98–105. doi: 10.1128/aem.46.1.98-105.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C. T., Patel R. N., Laskin A. I., Barnabe N., Marczak I. Identification and purification of a nicotinamide adenine dinucleotide-dependent secondary alcohol dehydrogenase from C1-utilizing microbes. FEBS Lett. 1979 May 1;101(1):179–183. doi: 10.1016/0014-5793(79)81321-6. [DOI] [PubMed] [Google Scholar]
- Hou C. T., Patel R., Barnabe N., Marczak I. Stereospecificity and other properties of a novel secondary-alcohol-specific alcohol dehydrogenase. Eur J Biochem. 1981 Oct;119(2):359–364. doi: 10.1111/j.1432-1033.1981.tb05616.x. [DOI] [PubMed] [Google Scholar]
- Hou C. T., Patel R., Laskin A. I., Barnabe N., Barist I. Epoxidation of short-chain alkenes by resting-cell suspensions of propane-grown bacteria. Appl Environ Microbiol. 1983 Jul;46(1):171–177. doi: 10.1128/aem.46.1.171-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C. T., Patel R., Laskin A. I., Barnabe N., Marczak I. Microbial oxidation of gaseous hydrocarbons: production of methyl ketones from their corresponding secondary alcohols by methane- and methanol-grown microbes. Appl Environ Microbiol. 1979 Jul;38(1):135–142. doi: 10.1128/aem.38.1.135-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C. T., Patel R., Laskin A. I., Barnabe N. Microbial oxidation of gaseous hydrocarbons: epoxidation of C2 to C4 n-alkenes by methylotrophic bacteria. Appl Environ Microbiol. 1979 Jul;38(1):127–134. doi: 10.1128/aem.38.1.127-134.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEADBETTER E. R., FOSTER J. W. Bacterial oxidation of gaseous alkanes. Arch Mikrobiol. 1960;35:92–104. doi: 10.1007/BF00425597. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamed R. J., Zeikus J. G. Novel NADP-linked alcohol--aldehyde/ketone oxidoreductase in thermophilic ethanologenic bacteria. Biochem J. 1981 Apr 1;195(1):183–190. doi: 10.1042/bj1950183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehaus W. G., Jr, Frielle T., Kingsley E. A., Jr Purification and characterization of a secondary alcohol dehydrogenase from a pseudomonad. J Bacteriol. 1978 Apr;134(1):177–183. doi: 10.1128/jb.134.1.177-183.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Hou C. T., Laskin A. I., Derelanko P., Felix A. Microbial production of methyl ketones. Purification and properties of a secondary alcohol dehydrogenase from yeast. Eur J Biochem. 1979 Nov;101(2):401–406. doi: 10.1111/j.1432-1033.1979.tb19732.x. [DOI] [PubMed] [Google Scholar]
- Patel R. N., Hou C. T., Laskin A. I., Felix A., Derelanko P. Microbial Oxidation of Gaseous Hydrocarbons: Production of Methylketones from Corresponding n-Alkanes by Methane-Utilizing Bacteria. Appl Environ Microbiol. 1980 Apr;39(4):727–733. doi: 10.1128/aem.39.4.727-733.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips W. E., Jr, Perry J. J. Metabolism of n-butane and 2-butanone by Mycobacterium vaccae. J Bacteriol. 1974 Nov;120(2):987–989. doi: 10.1128/jb.120.2.987-989.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestal J. R., Perry J. J. Divergent metabolic pathways for propane and propionate utilization by a soil isolate. J Bacteriol. 1969 Jul;99(1):216–221. doi: 10.1128/jb.99.1.216-221.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestal J. R., Perry J. J. Effect of substrate on the lipids of the hydrocarbon-utilizing Mycobacterium vaccae. Can J Microbiol. 1971 Apr;17(4):445–449. doi: 10.1139/m71-075. [DOI] [PubMed] [Google Scholar]
- Yoneya T., Sato Y. Alcohol dehydrogenase from Rhizopus javanicus. Appl Environ Microbiol. 1979 Jun;37(6):1073–1078. doi: 10.1128/aem.37.6.1073-1078.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


