Abstract
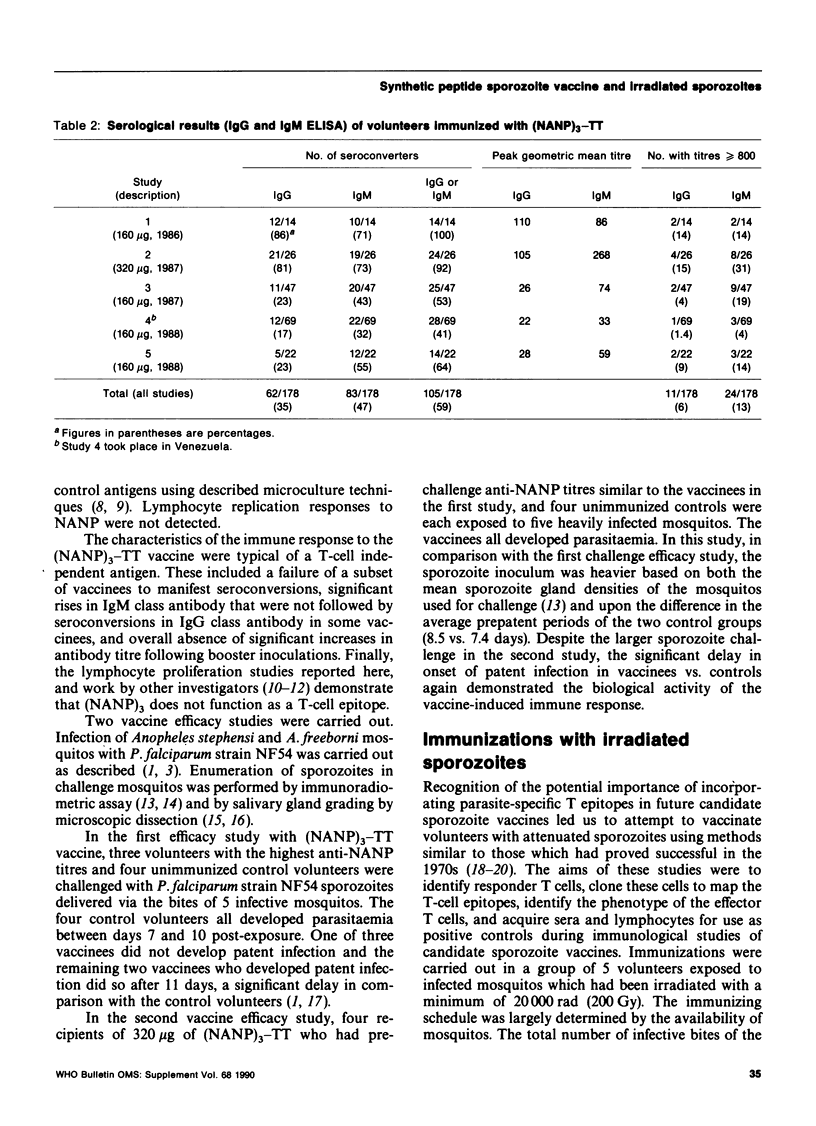
The synthetic peptide Plasmodium falciparum circumsporozoite (CS) protein conjugate vaccine (NANP)3-TT was safe when given parenterally to 202 volunteers. However, with a few notable exceptions, antibody responses were low and could not be boosted. Vaccinees' lymphocytes did not proliferate when exposed in vitro to (NANP)3. The tetanus toxoid (TT) carrier immunomodulated the response to the CS peptide in that both epitopic suppression and immune enhancement were demonstrated during the course of the clinical trials. During efficacy challenge studies, 1 of 7 vaccinees was protected against sporozoite challenge and in other vaccinees the prepatent period was significantly delayed. P. falciparum-infected mosquitos were irradiated with 20,000 rad (200 Gy). Five volunteers were immunized with 54, 55, 224, 663, and 715 total infective bites of irradiated mosquitos in an attempt to immunize with attenuated sporozoites. Four of these volunteers had significant humoral and cellular immune responses. Two volunteers (who received the largest immunizing doses) were challenged by the bites of infective mosquitos and both developed parasitaemia. In the volunteer with the highest antibody titre there was a marked delay in patency as determined by serial plasmodial cultures. T-cell clones are being obtained and characterized.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou W. R., Hoffman S. L., Sherwood J. A., Hollingdale M. R., Neva F. A., Hockmeyer W. T., Gordon D. M., Schneider I., Wirtz R. A., Young J. F. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet. 1987 Jun 6;1(8545):1277–1281. doi: 10.1016/s0140-6736(87)90540-x. [DOI] [PubMed] [Google Scholar]
- Campbell J. R., Paleologo F. P., Franke E. D., Ratiwayanto S., Hadiputranto H., Kurniawan L., Wistar R., Jr, Hoffman S. L., Annis B. A., Wasserman G. Immune response of humans to the circumsporozoite protein of Plasmodium falciparum: limited T cell response to the immunodominant central repeat region. Am J Trop Med Hyg. 1988 Sep;39(3):232–235. doi: 10.4269/ajtmh.1988.39.232. [DOI] [PubMed] [Google Scholar]
- Clyde D. F. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am J Trop Med Hyg. 1975 May;24(3):397–401. doi: 10.4269/ajtmh.1975.24.397. [DOI] [PubMed] [Google Scholar]
- Clyde D. F., Most H., McCarthy V. C., Vanderberg J. P. Immunization of man against sporozite-induced falciparum malaria. Am J Med Sci. 1973 Sep;266(3):169–177. doi: 10.1097/00000441-197309000-00002. [DOI] [PubMed] [Google Scholar]
- Collins W. E., Warren M., Skinner J. C., Chin W., Richardson B. B. Studies on the Santa Lucia (El Salvador) strain of Plasmodium falciparum in Aotus trivirgatus monkeys. J Parasitol. 1977 Feb;63(1):52–56. [PubMed] [Google Scholar]
- Davis J. R., Murphy J. R., Baqar S., Clyde D. F., Herrington D. A., Levine M. M. Estimate of anti-Plasmodium falciparum sporozoite activity in humans vaccinated with synthetic circumsporozoite protein (NANP)3. Trans R Soc Trop Med Hyg. 1989 Nov-Dec;83(6):748–750. doi: 10.1016/0035-9203(89)90315-5. [DOI] [PubMed] [Google Scholar]
- Davis J. R., Murphy J. R., Clyde D. F., Baqar S., Cochrane A. H., Zavala F., Nussenzweig R. S. Estimate of Plasmodium falciparum sporozoite content of Anopheles stephensi used to challenge human volunteers. Am J Trop Med Hyg. 1989 Feb;40(2):128–130. doi: 10.4269/ajtmh.1989.40.128. [DOI] [PubMed] [Google Scholar]
- Etlinger H. M., Felix A. M., Gillessen D., Heimer E. P., Just M., Pink J. R., Sinigaglia F., Stürchler D., Takacs B., Trzeciak A. Assessment in humans of a synthetic peptide-based vaccine against the sporozoite stage of the human malaria parasite, Plasmodium falciparum. J Immunol. 1988 Jan 15;140(2):626–633. [PubMed] [Google Scholar]
- Good M. F., Pombo D., Quakyi I. A., Riley E. M., Houghten R. A., Menon A., Alling D. W., Berzofsky J. A., Miller L. H. Human T-cell recognition of the circumsporozoite protein of Plasmodium falciparum: immunodominant T-cell domains map to the polymorphic regions of the molecule. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1199–1203. doi: 10.1073/pnas.85.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Herrington D., Murphy J. R., Morris J. G., Losonsky G., Tall B., Lindberg A. A., Svenson S., Baqar S., Edwards M. F. Safety, infectivity, immunogenicity, and in vivo stability of two attenuated auxotrophic mutant strains of Salmonella typhi, 541Ty and 543Ty, as live oral vaccines in humans. J Clin Invest. 1987 Mar;79(3):888–902. doi: 10.1172/JCI112899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Baqar S., Muñoz C., Schlesinger L., Ferreccio C., Lindberg A. A., Svenson S., Losonsky G., Koster F., Levine M. M. Characteristics of humoral and cellular immunity to Salmonella typhi in residents of typhoid-endemic and typhoid-free regions. J Infect Dis. 1987 Dec;156(6):1005–1009. doi: 10.1093/infdis/156.6.1005. [DOI] [PubMed] [Google Scholar]
- Nardin E., Gwadz R. W., Nussenzweig R. S. Characterization of sporozoite surface antigens by indirect immunofluorescence: detection of stage- and species-specific antimalarial antibodies. Bull World Health Organ. 1979;57 (Suppl 1):211–217. [PMC free article] [PubMed] [Google Scholar]
- Schutze M. P., Leclerc C., Jolivet M., Audibert F., Chedid L. Carrier-induced epitopic suppression, a major issue for future synthetic vaccines. J Immunol. 1985 Oct;135(4):2319–2322. [PubMed] [Google Scholar]
- Schutze M. P., Leclerc C., Vogel F. R., Chedid L. Epitopic suppression in synthetic vaccine models: analysis of the effector mechanisms. Cell Immunol. 1987 Jan;104(1):79–90. doi: 10.1016/0008-8749(87)90008-6. [DOI] [PubMed] [Google Scholar]
- Sinigaglia F., Guttinger M., Gillessen D., Doran D. M., Takacs B., Matile H., Trzeciak A., Pink J. R. Epitopes recognized by human T lymphocytes on malaria circumsporozoite protein. Eur J Immunol. 1988 Apr;18(4):633–636. doi: 10.1002/eji.1830180422. [DOI] [PubMed] [Google Scholar]
- Zavala F., Gwadz R. W., Collins F. H., Nussenzweig R. S., Nussenzweig V. Monoclonal antibodies to circumsporozoite proteins identify the species of malaria parasite in infected mosquitoes. Nature. 1982 Oct 21;299(5885):737–738. doi: 10.1038/299737a0. [DOI] [PubMed] [Google Scholar]


