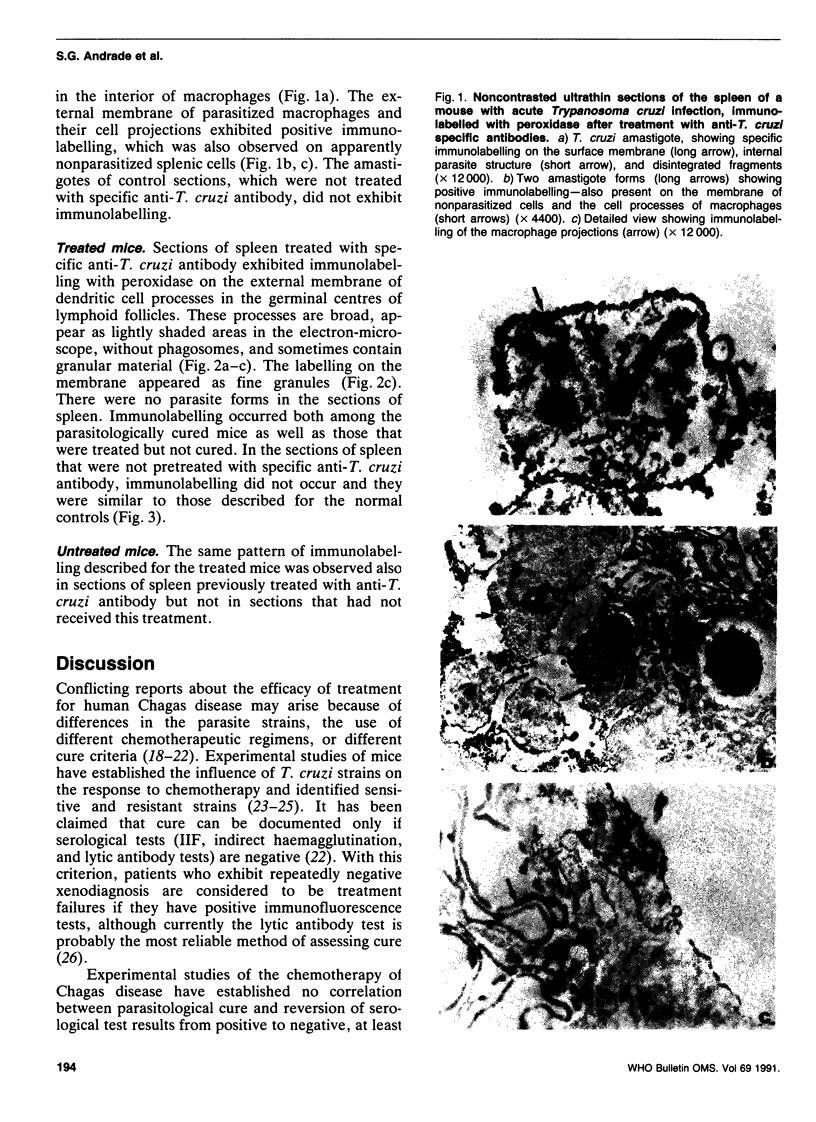
Abstract
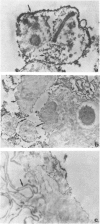
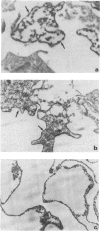
Mice infected with Trypanosoma cruzi, but parasitologically cured after specific chemotherapy, continued to exhibit positive indirect immunofluorescence serological tests 3-6 months after the therapy. Treatment of trypanosome antigens with monospecific antisera produced in rabbits, and examination by immunoelectron-microscopy following peroxidase labelling disclosed the presence of membrane deposits in cell processes in the spleens of the mice. Similar deposits were observed in the external membranes of T. cruzi amastigotes in the spleens of acutely infected mice, but not in normal control mice. No reaction occurred in tissues not previously treated with the monospecific anti-T. cruzi serum. Positive cells in treated and cured mice, as well as in the not cured or untreated control mice, were located in germinal centres of the splenic white pulp and presented long and branching cytoplasmic processes, which are indicative of dendritic cells of the lymphoid follicles of the spleen.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade S. G., Freitas L. A., Peyrol S., Pimentel A. R., Sadigursky M. Trypanosoma cruzi antigens detected by immunoelectron microscopy in the spleen of mice serologically positive but parasitologically cured by chemotherapy. (Preliminary report). Rev Soc Bras Med Trop. 1988 Jan-Mar;21(1):41–42. doi: 10.1590/s0037-86821988000100010. [DOI] [PubMed] [Google Scholar]
- Andrade S. G., Magalhães J. B., Pontes A. L. Evaluation of chemotherapy with benznidazole and nifurtimox in mice infected with Trypanosoma cruzi strains of different types. Bull World Health Organ. 1985;63(4):721–726. [PMC free article] [PubMed] [Google Scholar]
- Andrade S. G., Silva R. C., Santiago C. M., Freitas L. A. Therapeutic action of MK-436 (2,5-nitroimidazole) on Trypanosoma cruzi infections in mice: a parasitological, serological, histopathological, and ultrastructural study. Bull World Health Organ. 1987;65(5):625–633. [PMC free article] [PubMed] [Google Scholar]
- Andrade S. G., Silva R. C., Santiago C. M. Treatment of chronic experimental Trypanosoma cruzi infections in mice with MK-436, a 2-substituted 5-nitroimidazole. Bull World Health Organ. 1989;67(5):509–514. [PMC free article] [PubMed] [Google Scholar]
- Apt W. Tratamiento de la enfermedad de Chagas. Rev Med Chil. 1985 Feb;113(2):162–166. [PubMed] [Google Scholar]
- Brener Z., Costa C. A., Chiari C. Differences in the susceptibility of Trypanosoma cruzi strains to active chemotherapeutic agents. Rev Inst Med Trop Sao Paulo. 1976 Nov-Dec;18(6):450–455. [PubMed] [Google Scholar]
- Brown J. C., De Jesus D. G., Holborow E. J., Harris G. Lymphocyte-mediated transport of aggregated human gamma-globulin into germinal centre areas of normal mouse spleen. Nature. 1970 Oct 24;228(5269):367–369. doi: 10.1038/228367a0. [DOI] [PubMed] [Google Scholar]
- Camargo M. E. Fluorescent antibody test for the serodiagnosis of American trypanosomiasis. Technical modification employing preserved culture forms of Trypanosoma cruzi in a slide test. Rev Inst Med Trop Sao Paulo. 1966 Sep-Oct;8(5):227–235. [PubMed] [Google Scholar]
- Filardi L. S., Brener Z. A nitroimidazole-thiadiazole derivative with curative action in experimental Trypanosoma cruzi infections. Ann Trop Med Parasitol. 1982 Jun;76(3):293–297. doi: 10.1080/00034983.1982.11687544. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Hanna M. G., Jr, Szakal A. K. Localization of 125I-labeled antigen in germinal centers of mouse spleen: histologic and ultrastructural autoradiographic studies of the secondary immune reaction. J Immunol. 1968 Nov;101(5):949–962. [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Nakane P K, Pierce G B., Jr Enzyme-labeled antibodies: preparation and application for the localization of antigens. J Histochem Cytochem. 1966 Dec;14(12):929–931. doi: 10.1177/14.12.929. [DOI] [PubMed] [Google Scholar]
- Neal R. A., van Bueren J. Comparative studies of drug susceptibility of five strains of Trypanosoma cruzi in vivo and in vitro. Trans R Soc Trop Med Hyg. 1988;82(5):709–714. doi: 10.1016/0035-9203(88)90208-8. [DOI] [PubMed] [Google Scholar]
- Nossal G. J., Abbot A., Mitchell J., Lummus Z. Antigens in immunity. XV. Ultrastructural features of antigen capture in primary and secondary lymphoid follicles. J Exp Med. 1968 Feb 1;127(2):277–290. doi: 10.1084/jem.127.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tew J. G., Mandel T. The maintenance and regulation of serum antibody levels: evidence indicating a role for antigen retained in lymphoid follicles. J Immunol. 1978 Mar;120(3):1063–1069. [PubMed] [Google Scholar]
- Van Rooijen N. Binding of labelled antigens and immune complexes to macrophages and dendritic cells in cryostat sections of normal mouse spleen. Acta Morphol Neerl Scand. 1978 May;16(2):121–127. [PubMed] [Google Scholar]