Abstract
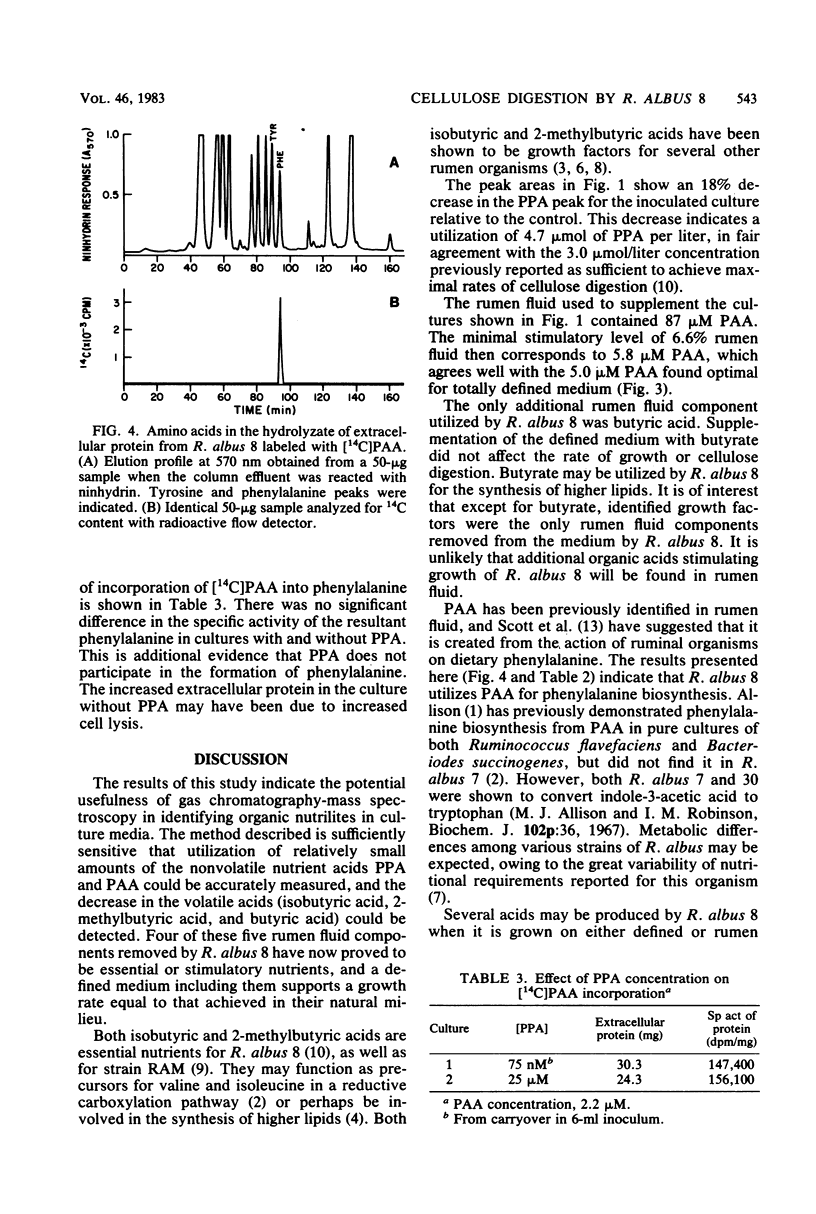
The rate of cellulose digestion by Ruminococcus albus 8 grown on a defined medium could be increased by adding a minimum of 6.6% (vol/vol) rumen fluid. Strain 8 was grown on half this concentration, and the culture medium before and after growth was analyzed by gas chromatography-mass spectrometry to determine which components of the rumen fluid were used. Phenylacetic acid was identified as the component needed to make the defined medium nutritionally equivalent to one supplemented with rumen fluid. [14C]phenylacetic acid fed to cultures of strain 8 was primarily incorporated into protein. Hydrolysis of protein samples and separation of the resulting amino acids showed that only phenylalanine was labeled. The results indicate that cellulose digestion by strain 8 was probably limited by phenylalanine biosynthesis in our previously reported medium. The data obtained on the utilization of other rumen fluid components, as well as on the production of metabolites, illustrate the potential usefulness of this method in formulating defined media to simulate those in nature.
Full text
PDF

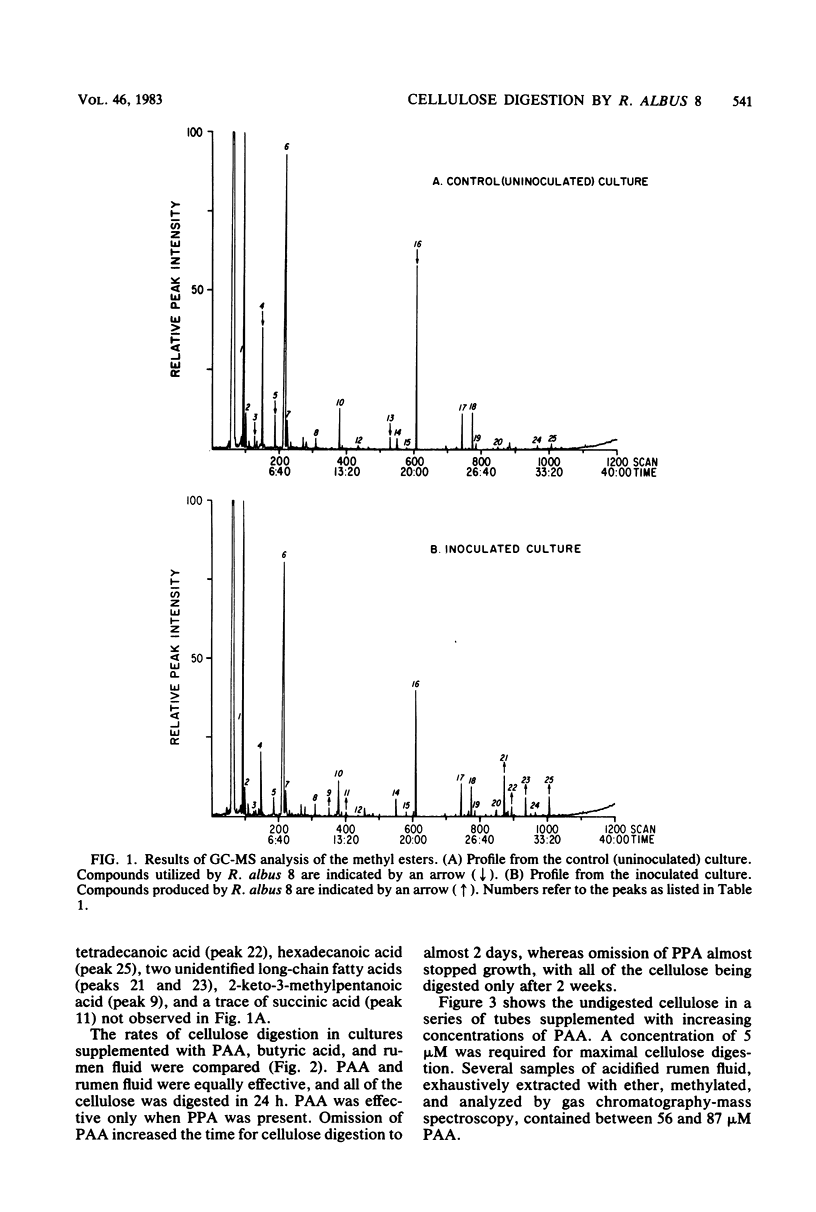
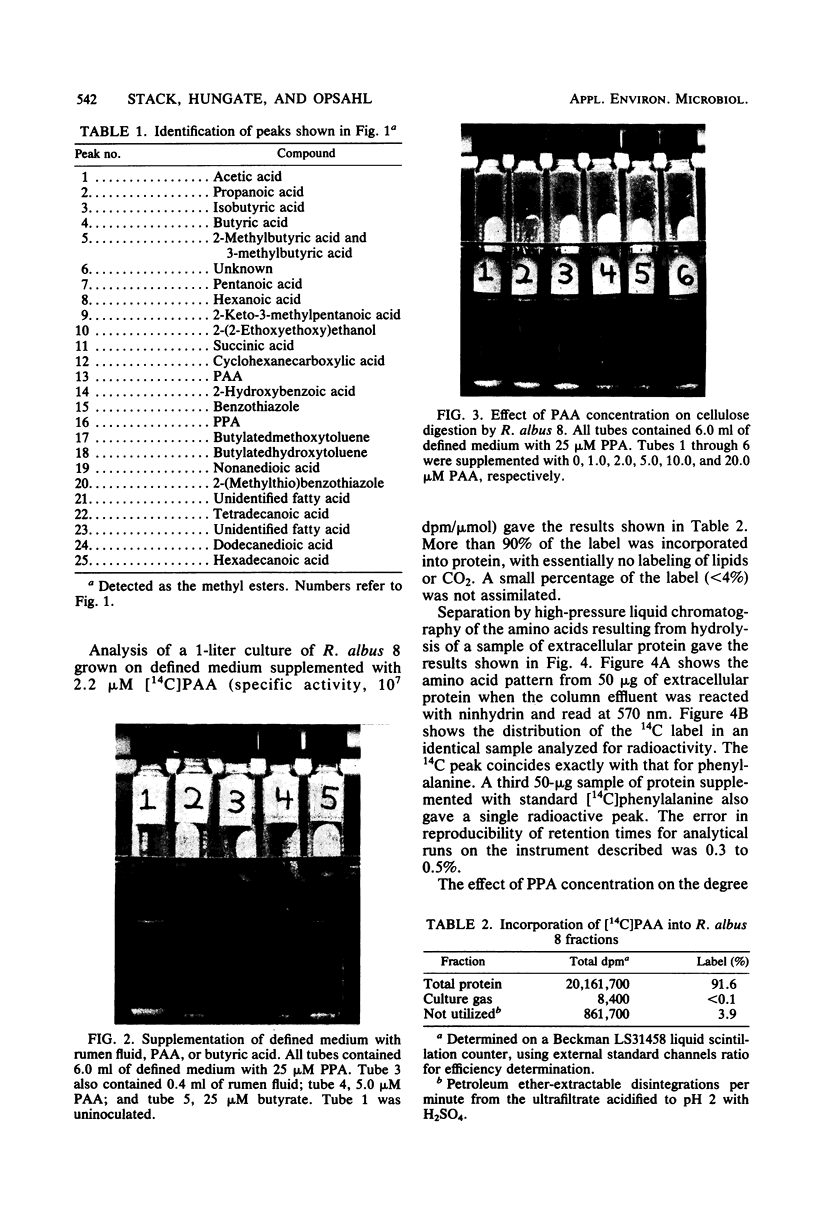

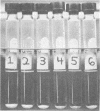
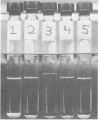
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON M. J., BRYANT M. P., DOETSCH R. N. Volatile fatty acid growth factor for cellulolytic cocci of bovine rumen. Science. 1958 Aug 29;128(3322):474–475. doi: 10.1126/science.128.3322.474. [DOI] [PubMed] [Google Scholar]
- ALLISON M. J., BRYANT M. P., KATZ I., KEENEY M. Metabolic function of branched-chain volatile fatty acids, growth factors for ruminococci. II. Biosynthesis of higher branched-chain fatty acids and aldehydes. J Bacteriol. 1962 May;83:1084–1093. doi: 10.1128/jb.83.5.1084-1093.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLISON M. J. PHENYLALANINE BIOSYNTHESIS FROM PHENYLACETIC ACID BY ANAEROBIC BACTERIA FROM THE RUMEN. Biochem Biophys Res Commun. 1965 Jan 4;18:30–35. doi: 10.1016/0006-291x(65)90877-6. [DOI] [PubMed] [Google Scholar]
- Allison M. J. Biosynthesis of amono acids by ruminal microorganisms. J Anim Sci. 1969 Nov;29(5):797–807. doi: 10.2527/jas1969.295797x. [DOI] [PubMed] [Google Scholar]
- BRYANT M. P., ROBINSON I. M. Some nutritional characteristics of predominant culturable ruminal bacteria. J Bacteriol. 1962 Oct;84:605–614. doi: 10.1128/jb.84.4.605-614.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bryant M. P. Nutritional requirements of the predominant rumen cellulolytic bacteria. Fed Proc. 1973 Jul;32(7):1809–1813. [PubMed] [Google Scholar]
- Bryant M. P., Robinson I. M. Some Nutritional Requirements of the Genus Ruminococcus. Appl Microbiol. 1961 Mar;9(2):91–95. doi: 10.1128/am.9.2.91-95.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. POLYSACCHARIDE STORAGE AND GROWTH EFFICIENCY IN RUMINOCOCCUS ALBUS. J Bacteriol. 1963 Oct;86:848–854. doi: 10.1128/jb.86.4.848-854.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungate R. E., Stack R. J. Phenylpropanoic Acid: Growth Factor for Ruminococcus albus. Appl Environ Microbiol. 1982 Jul;44(1):79–83. doi: 10.1128/aem.44.1.79-83.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre M., Rucker R. B. Aorta elastin turnover in normal and hypercholesterolemic Japanese quail. Biochim Biophys Acta. 1980 Jul 15;630(4):519–529. doi: 10.1016/0304-4165(80)90006-9. [DOI] [PubMed] [Google Scholar]
- Petersen M. C., Vine J., Ashley J. J., Nation R. L. Leaching of 2-(2-hydroxyethylmercapto) benzothiazole into contents of disposable syringes. J Pharm Sci. 1981 Oct;70(10):1139–1143. doi: 10.1002/jps.2600701012. [DOI] [PubMed] [Google Scholar]
- Scott T. W., Ward P. F., Dawson R. M. The formation and metabolism of phenyl-substituted fatty acids in the ruminant. Biochem J. 1964 Jan;90(1):12–24. doi: 10.1042/bj0900012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS C. M. Gas chromatography of urinary aromatic acids. Anal Biochem. 1962 Dec;4:423–432. doi: 10.1016/0003-2697(62)90124-0. [DOI] [PubMed] [Google Scholar]