Abstract
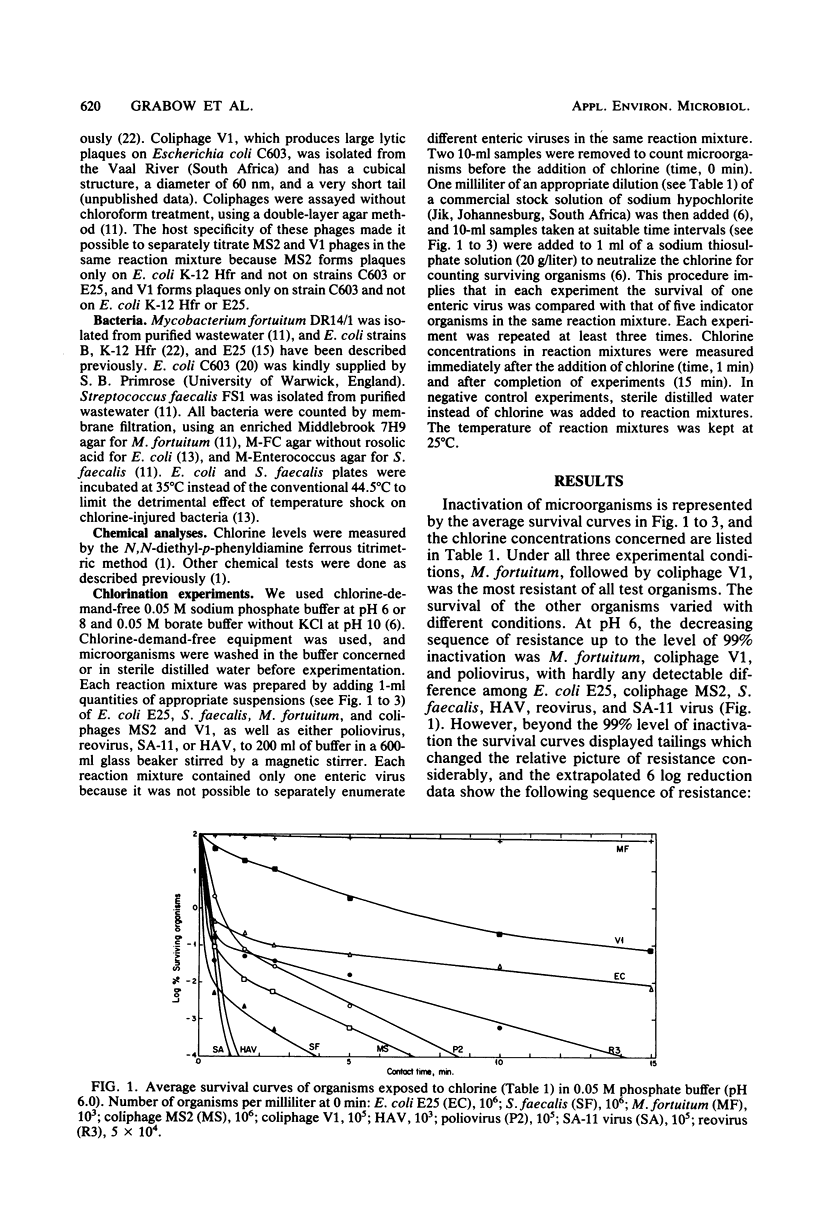
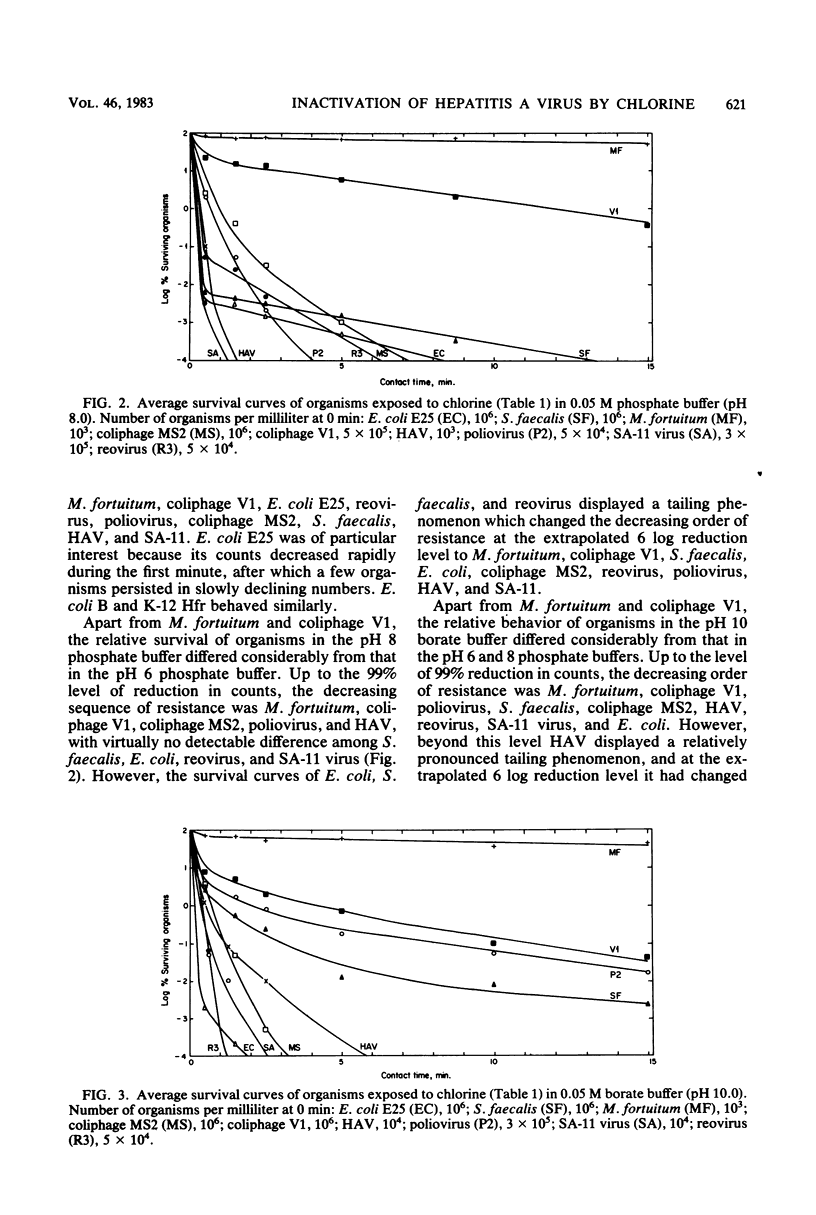
Hepatitis A virus (HAV) and selected indicator organisms were mixed together in chlorine-demand-free buffers at pH 6, 8, or 10 and exposed to free chlorine residuals, and the survival kinetics of individual organisms were compared. HAV was enumerated by a most-probable-number dilution assay, using PLC/PRF/5 liver cells for propagation of the virus and radioimmunoassay for its detection. At all pH levels, HAV was more sensitive than Mycobacterium fortuitum, coliphage V1 (representing a type of phage common in some sewage-polluted waters), and poliovirus type 2. Under certain conditions, HAV was more resistant than Escherichia coli, Streptococcus faecalis, coliphage MS2, and reovirus type 3. It was always more resistant than SA-11 rotavirus. Evidence is presented that conditions generally specified for the chlorine disinfection of drinking-water supplies will also successfully inactivate HAV and that HAV inactivation by free chlorine residuals can reliably be monitored by practical indicator systems consisting of appropriate combinations of suitable indicators such as coliform and acid-fast bacteria, coliphages, the standard plate count, and fecal streptococci.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg G., Dahling D. R., Brown G. A., Berman D. Validity of fecal coliforms, total coliforms, and fecal streptococci as indicators of viruses in chlorinated primary sewage effluents. Appl Environ Microbiol. 1978 Dec;36(6):880–884. doi: 10.1128/aem.36.6.880-884.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daemer R. J., Feinstone S. M., Gust I. D., Purcell R. H. Propagation of human hepatitis A virus in African green monkey kidney cell culture: primary isolation and serial passage. Infect Immun. 1981 Apr;32(1):388–393. doi: 10.1128/iai.32.1.388-393.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelbrecht R. S., Weber M. J., Salter B. L., Schmidt C. A. Comparative inactivation of viruses by chlorine. Appl Environ Microbiol. 1980 Aug;40(2):249–256. doi: 10.1128/aem.40.2.249-256.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frösner G. G., Deinhardt F., Scheid R., Gauss-Müller V., Holmes N., Messelberger V., Siegl G., Alexander J. J. Propagation of human hepatitis A virus in a hepatoma cell line. Infection. 1979;7(6):303–305. doi: 10.1007/BF01642154. [DOI] [PubMed] [Google Scholar]
- Gauss-Müller V., Frösner G. G., Deinhardt F. Propagation of hepatitis A virus in human embryo fibroblasts. J Med Virol. 1981;7(3):233–239. doi: 10.1002/jmv.1890070308. [DOI] [PubMed] [Google Scholar]
- Grabow W. O., Du Randt W. C., Prozesky O. W., Scott W. E. Microcystis aeruginosa toxin: cell culture toxicity, hemolysis, and mutagenicity assays. Appl Environ Microbiol. 1982 Jun;43(6):1425–1433. doi: 10.1128/aem.43.6.1425-1433.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabow W. O., Prozesky O. W. Drug resistance of coliform bacteria in hospital and city sewage. Antimicrob Agents Chemother. 1973 Feb;3(2):175–180. doi: 10.1128/aac.3.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H., Shibayama T., Sato A., Suzuki S., Ichida F., Hamada C. Propagation of human hepatitis A virus in conventional cell lines. J Med Virol. 1981;7(4):273–286. doi: 10.1002/jmv.1890070404. [DOI] [PubMed] [Google Scholar]
- Logan K. B., Scott G. E., Seeley N. D., Primrose S. B. A portable device for the rapid concentration of viruses from large volumes of natural freshwater. J Virol Methods. 1981 Nov;3(4):241–249. doi: 10.1016/0166-0934(81)90074-4. [DOI] [PubMed] [Google Scholar]
- Neefe J. R., Baty J. B., Reinhold J. G., Stokes J. Inactivation of the Virus of Infectious Hepatitis in Drinking Water. Am J Public Health Nations Health. 1947 Apr;37(4):365–372. [PMC free article] [PubMed] [Google Scholar]
- Peterson D. A., Hurley T. R., Hoff J. C., Wolfe L. G. Effect of chlorine treatment on infectivity of hepatitis A virus. Appl Environ Microbiol. 1983 Jan;45(1):223–227. doi: 10.1128/aem.45.1.223-227.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provost P. J., Giesa P. A., McAleer W. J., Hilleman M. R. Isolation of hepatitis A virus in vitro in cell culture directly from human specimens. Proc Soc Exp Biol Med. 1981 Jun;167(2):201–206. doi: 10.3181/00379727-167-41149. [DOI] [PubMed] [Google Scholar]
- Ridgway H. F., Olson B. H. Chlorine resistance patterns of bacteria from two drinking water distribution systems. Appl Environ Microbiol. 1982 Oct;44(4):972–987. doi: 10.1128/aem.44.4.972-987.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl G., Frösner G. G., Gauss-Müller V., Tratschin J. D., Deinhardt F. The physicochemical properties of infectious hepatitis A virions. J Gen Virol. 1981 Dec;57(Pt 2):331–341. doi: 10.1099/0022-1317-57-2-331. [DOI] [PubMed] [Google Scholar]
- Tratschin J. D., Siegl G., Frösner G. G., Deinhardt F. Characterization and classification of virus particles associated with hepatitis A. III. Structural proteins. J Virol. 1981 Apr;38(1):151–156. doi: 10.1128/jvi.38.1.151-156.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


