Abstract
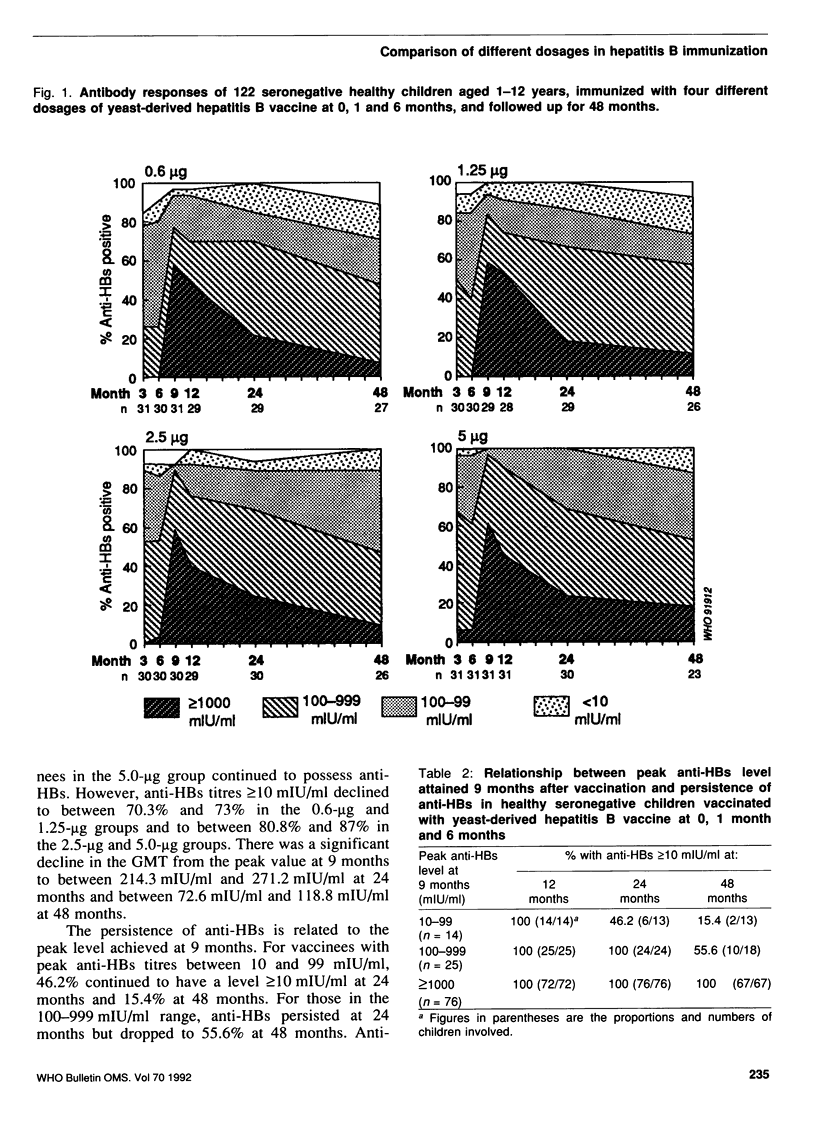
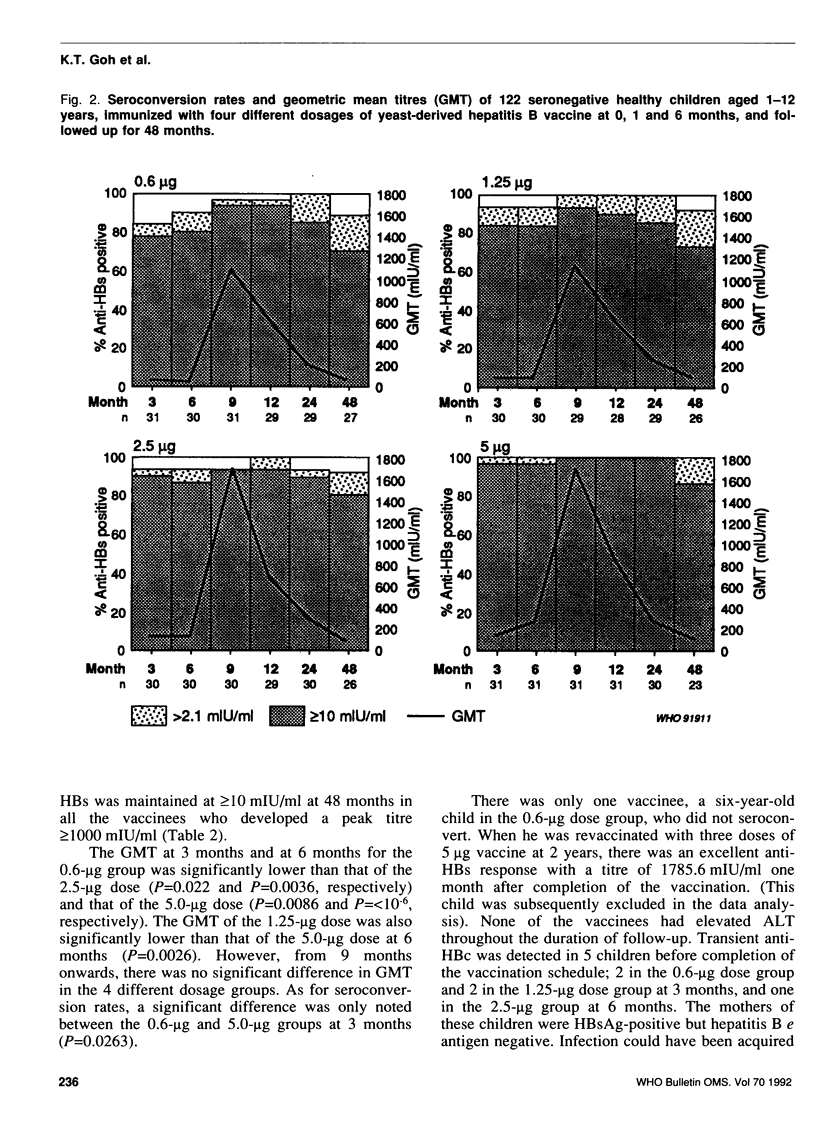
The immunogenicity of four different dosages of yeast-derived hepatitis B vaccine (Merck, Sharp & Dohme: 0.6 micrograms, 1.25 micrograms, 2.5 micrograms and 5.0 micrograms), administered at 0, 1 and 6 months (0-1-6 schedule) intramuscularly, was evaluated in 122 seronegative healthy children 1-12 years of age. Three months after the first dose, 83.9-100% of the vaccinees seroconverted. Peak geometric mean titres (GMT) of between 1088 mlU/ml and 1699 mlU/ml were attained 3 months after completion of the vaccination schedule. After 24 months, anti-HBs (antibody to hepatitis B surface antigen) was detected in 93.1-100% of the vaccinees, but the GMT dropped to between 214.3 mlU/ml and 303.5 mlU/ml. After 48 months, 88.8-100% of the vaccinees continued to possess anti-HBs and 70.3-87% had titres above 10 mlU/ml. As expected, the GMT declined further to between 72.6 mlU/ml and 118.8 mlU/ml. There were no significant differences in seroconversion rates and GMT among the different dosage groups. All the vaccinees remained asymptomatic and free from hepatitis B virus infection. The study showed that reduced dosages of the vaccine (0.6 micrograms, 1.25 micrograms and 2.5 micrograms) were as immunogenic as the standard dose (5 micrograms); the 2.5-micrograms dose was recommended for the national childhood immunization programme in Singapore. No booster is necessary for at least four years after vaccination.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coursaget P., Yvonnet B., Chotard J., Sarr M., Vincelot P., N'doye R., Diop-Mar I., Chiron J. P. Seven-year study of hepatitis B vaccine efficacy in infants from an endemic area (Senegal). Lancet. 1986 Nov 15;2(8516):1143–1145. doi: 10.1016/s0140-6736(86)90543-x. [DOI] [PubMed] [Google Scholar]
- Dandolos E., Roumeliotou-Karayannis A., Richardson S. C., Papaevangelou G. Safety and immunogenicity of a recombinant hepatitis B vaccine. J Med Virol. 1985 Sep;17(1):57–62. doi: 10.1002/jmv.1890170109. [DOI] [PubMed] [Google Scholar]
- Francis D. P., Feorino P. M., McDougal S., Warfield D., Getchell J., Cabradilla C., Tong M., Miller W. J., Schultz L. D., Bailey F. J. The safety of the hepatitis B vaccine. Inactivation of the AIDS virus during routine vaccine manufacture. JAMA. 1986 Aug 15;256(7):869–872. [PubMed] [Google Scholar]
- Goh K. T., Doraisingham S., Tan K. L., Oon C. J., Ho M. L., Chen A. J., Chan S. H. The hepatitis B immunization programme in Singapore. Bull World Health Organ. 1989;67(1):65–70. [PMC free article] [PubMed] [Google Scholar]
- Guan R., Tay H. H., Yap I., Smith R., Tan L. H. Immunogenicity of a low dose recombinant DNA hepatitis B vaccine in healthy adults in Singapore. Asian Pac J Allergy Immunol. 1989 Dec;7(2):85–88. [PubMed] [Google Scholar]
- Guan R., Tay H. H., Yap I., Smith R., Tan L. H. The immune response of low dose recombinant DNA hepatitis B vaccine in teenagers in Singapore. Trans R Soc Trop Med Hyg. 1990 Sep-Oct;84(5):731–732. doi: 10.1016/0035-9203(90)90167-d. [DOI] [PubMed] [Google Scholar]
- Hadler S. C., Francis D. P., Maynard J. E., Thompson S. E., Judson F. N., Echenberg D. F., Ostrow D. G., O'Malley P. M., Penley K. A., Altman N. L. Long-term immunogenicity and efficacy of hepatitis B vaccine in homosexual men. N Engl J Med. 1986 Jul 24;315(4):209–214. doi: 10.1056/NEJM198607243150401. [DOI] [PubMed] [Google Scholar]
- Jilg W., Deinhardt F. Results of immunisation with a recombinant yeast-derived hepatitis B vaccine. J Infect. 1986 Jul;13 (Suppl A):47–51. doi: 10.1016/s0163-4453(86)92683-6. [DOI] [PubMed] [Google Scholar]
- Jilg W., Schmidt M., Deinhardt F., Zachoval R. Hepatitis B vaccination: how long does protection last? Lancet. 1984 Aug 25;2(8400):458–458. doi: 10.1016/s0140-6736(84)92926-x. [DOI] [PubMed] [Google Scholar]
- McAleer W. J., Buynak E. B., Maigetter R. Z., Wampler D. E., Miller W. J., Hilleman M. R. Human hepatitis B vaccine from recombinant yeast. Nature. 1984 Jan 12;307(5947):178–180. doi: 10.1038/307178a0. [DOI] [PubMed] [Google Scholar]
- Milne A., Moyes C. D., Allwood G. K., Pearce N. E., Krugman S. Antibody responses to recombinant, yeast-derived hepatitis B vaccine in teenage New Zealand children. N Z Med J. 1988 Feb 24;101(840):67–69. [PubMed] [Google Scholar]
- Oon C. J., Guan R., Wong-Yong L. Y., Smith R. Clinical evaluation of a yeast recombinant hepatitis B vaccine in healthy hospital staff in Singapore. Ann Acad Med Singapore. 1988 Apr;17(2):185–189. [PubMed] [Google Scholar]
- Oon C. J., Tan K. L., Goh K. T., Wong-Yong L., Viegas O., McCarthy T., Chan S. H., Lee H. P. Evaluation of a low dose of hepatitis B vaccine given within a childhood immunisation programme in Singapore. J Infect. 1986 Nov;13(3):255–267. doi: 10.1016/s0163-4453(86)91223-5. [DOI] [PubMed] [Google Scholar]
- Stevens C. E., Taylor P. E., Tong M. J., Toy P. T., Vyas G. N., Nair P. V., Weissman J. Y., Krugman S. Yeast-recombinant hepatitis B vaccine. Efficacy with hepatitis B immune globulin in prevention of perinatal hepatitis B virus transmission. JAMA. 1987 May 15;257(19):2612–2616. doi: 10.1001/jama.257.19.2612. [DOI] [PubMed] [Google Scholar]


