Abstract
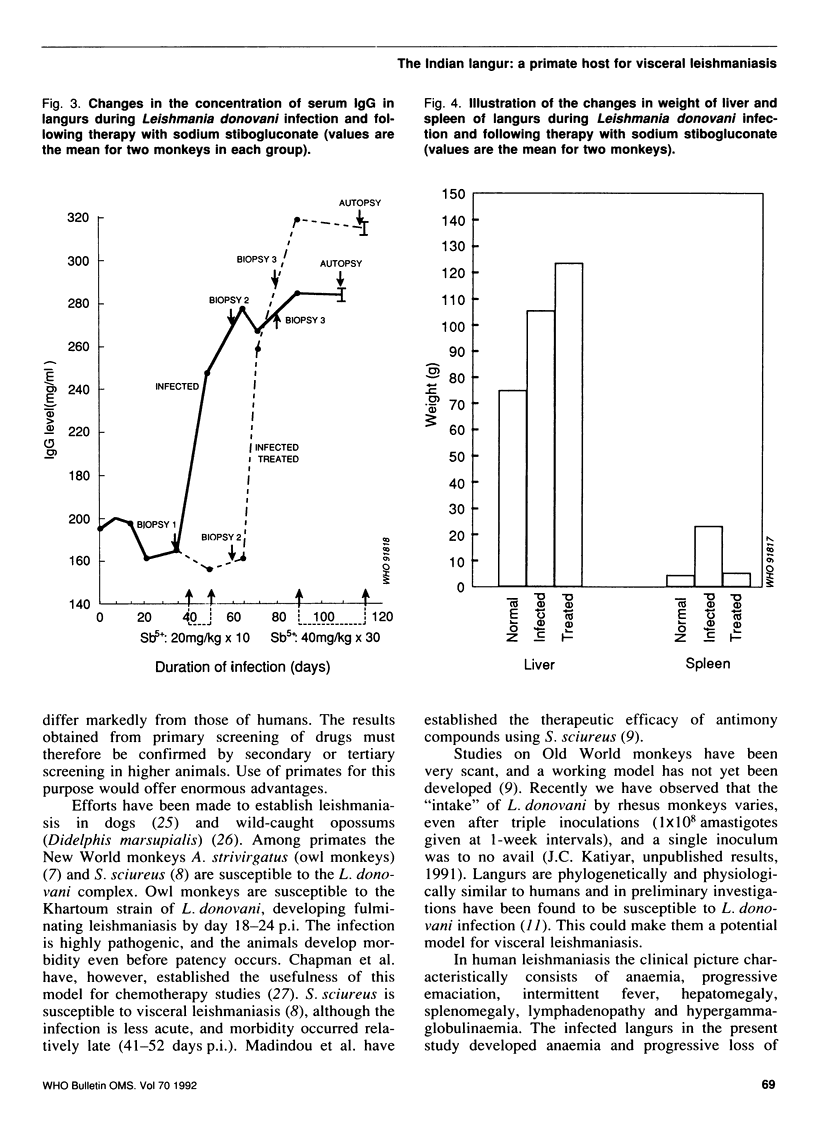
Described are the susceptibility of the Indian langur (Presbytis entellus) to Leishmania donovani and the consequent haematological and serum biochemical changes. The host response to antileishmanial chemotherapy and the immunological profile were also examined. Each langur was inoculated intravenously with 1 x 10(8) amastigotes; a spleen biopsy carried out on day 35 post-infection (p.i.) revealed 10-13 L. donovani bodies per 500 cell nuclei, which reached a maximum of 130-195 at death (day 105-110 p.i.). The infected monkeys lost body weight, developed severe anaemia, lymphocytosis, hyperproteinaemia, hypergammaglobulinaemia, hypoalbuminaemia and an increase in the level of alkaline phosphatase and alanine aminotransferase (AAT). Treatment with sodium stibogluconate (60 mg Sb5+ per kg body weight intramuscularly for 10 days) reduced the number of spleen parasites (0-1 amastigotes per 500 cell nuclei) but after the therapy the parasites appeared in the skin, which had previously been free of infection. Relapse occurred on day 30 post-treatment (10-24 amastigotes per 500 cell nuclei) and the parasites were resistant to repeat intensive therapy (120 mg Sb5+ per kg per day x 30 days). The stibogluconate treatment caused a proportionate reduction in the haematological and biochemical parameters to normal values except for alkaline phosphatase and AAT, which remained elevated. The level of IgG antibodies, which rose during the infection, rapidly fell to the pretreatment value following the first therapeutic schedule and then increased a second time coinciding with relapse. Our findings suggest that langurs could serve as acceptable models for human visceral leishmaniasis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowers G. N., Jr, McComb R. B. Measurement of total alkaline phosphatase activity in human serum. Clin Chem. 1975 Dec;21(13):1988–1995. [PubMed] [Google Scholar]
- CARTWRIGHT G. E., CHUNG H. L., CHANG A. Studies on the pancytopenia of kala-azar. Blood. 1948 Mar;3(3):249–275. [PubMed] [Google Scholar]
- Chapman W. L., Jr, Hanson W. L., Hendricks L. D. Leishmania donovani in the owl monkey (aotus trivirgatus) Trans R Soc Trop Med Hyg. 1981;75(1):124–125. doi: 10.1016/0035-9203(81)90032-8. [DOI] [PubMed] [Google Scholar]
- Chapman W. L., Jr, Hanson W. L., Hendricks L. D. Toxicity and efficacy of the antileishmanial drug meglumine antimoniate in the owl monkey (Aotus trivirgatus). J Parasitol. 1983 Dec;69(6):1176–1177. [PubMed] [Google Scholar]
- Chapman W. L., Jr, Hanson W. L. Visceral leishmaniasis in the squirrel monkey (Saimiri sciurea). J Parasitol. 1981 Oct;67(5):740–741. [PubMed] [Google Scholar]
- Chapman W. L., Jr, Hanson W. L., Waits V. B., Kinnamon K. E. Antileishmanial activity of selected compounds in dogs experimentally infected with Leishmania donovani. Rev Inst Med Trop Sao Paulo. 1979 Jul-Aug;21(4):189–193. [PubMed] [Google Scholar]
- Cooper R. W. Small species of primates in biomedical research. Lab Anim Care. 1968 Apr;18(2 Suppl):267–279. [PubMed] [Google Scholar]
- Dennis V. A., Chapman W. L., Jr, Hanson W. L., Lujan R. Leishmania donovani: clinical, hematologic and hepatic changes in squirrel monkeys (Saimiri sciureus). J Parasitol. 1985 Oct;71(5):576–582. [PubMed] [Google Scholar]
- Freireich E. J., Gehan E. A., Rall D. P., Schmidt L. H., Skipper H. E. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966 May;50(4):219–244. [PubMed] [Google Scholar]
- Haldar J. P., Ghose S., Saha K. C., Ghose A. C. Cell-mediated immune response in Indian kala-azar and post-kala-azar dermal leishmaniasis. Infect Immun. 1983 Nov;42(2):702–707. doi: 10.1128/iai.42.2.702-707.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson W. L., Chapman W. L., Jr, Hendricks L. D. Leishmania donovani in the oppossum (Didelphis marsupialis). J Parasitol. 1980 Aug;66(4):700–701. [PubMed] [Google Scholar]
- Hanson W. L., Chapman W. L., Jr, Kinnamon K. E. Testing of drugs for antileishmanial activity in golden hamsters infected with Leishmania donovani. Int J Parasitol. 1977 Dec;7(6):443–447. doi: 10.1016/0020-7519(77)90004-2. [DOI] [PubMed] [Google Scholar]
- Madindou T. J., Hanson W. L., Chapman W. L., Jr Chemotherapy of visceral leishmaniasis (Leishmania donovani) in the squirrel monkey (Saimiri sciureus). Ann Trop Med Parasitol. 1985 Feb;79(1):13–19. doi: 10.1080/00034983.1985.11811884. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Manson-Bahr P. E. Leishmaniasis. Int Rev Trop Med. 1971;4:123–140. [PubMed] [Google Scholar]
- Marsden P. D., Cuba C. C., Vexenat A., Costa e Silva M., Costa e Silva A., Barreto A. C. Experimental Leishmania chagasi infections in the marmoset Callithrix jacchus jacchus. Trans R Soc Trop Med Hyg. 1981;75(2):314–315. doi: 10.1016/0035-9203(81)90347-3. [DOI] [PubMed] [Google Scholar]
- SATI M. H. A NEW EXPERIMENTAL HOST OF LEISHMANIA DONOVANI. Exp Parasitol. 1963 Aug;14:52–53. doi: 10.1016/0014-4894(63)90009-2. [DOI] [PubMed] [Google Scholar]
- Thakur C. P., Kumar M., Kumar P., Mishra B. N., Pandey A. K. Rationalisation of regimens of treatment of kala-azar with sodium stibogluconate in India: a randomised study. Br Med J (Clin Res Ed) 1988 Jun 4;296(6636):1557–1561. doi: 10.1136/bmj.296.6636.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinder P., Kirkland J. F. The determination of L-aspartate:2-oxoglutarate aminotransferase in serum. Clin Chim Acta. 1967 May;16(2):287–296. doi: 10.1016/0009-8981(67)90194-5. [DOI] [PubMed] [Google Scholar]
- WEBSTER D. The determination of total and ester cholesterol in whole blood, serum or plasma. Clin Chim Acta. 1962 Mar;7:277–284. doi: 10.1016/0009-8981(62)90021-9. [DOI] [PubMed] [Google Scholar]
- Wyler D. J., Weinbaum F. I., Herrod H. R. Characterization of in vitro proliferative responses of human lymphocytes to leishmanial antigens. J Infect Dis. 1979 Aug;140(2):215–221. doi: 10.1093/infdis/140.2.215. [DOI] [PubMed] [Google Scholar]