Abstract
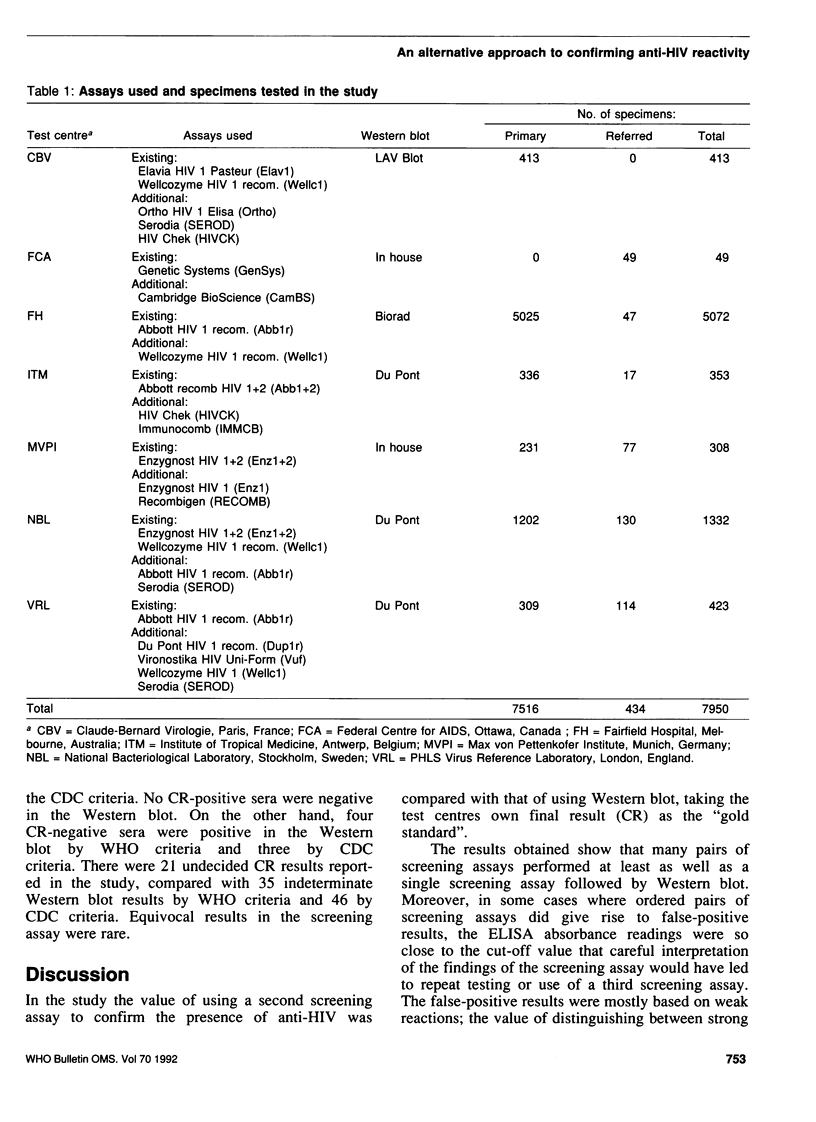
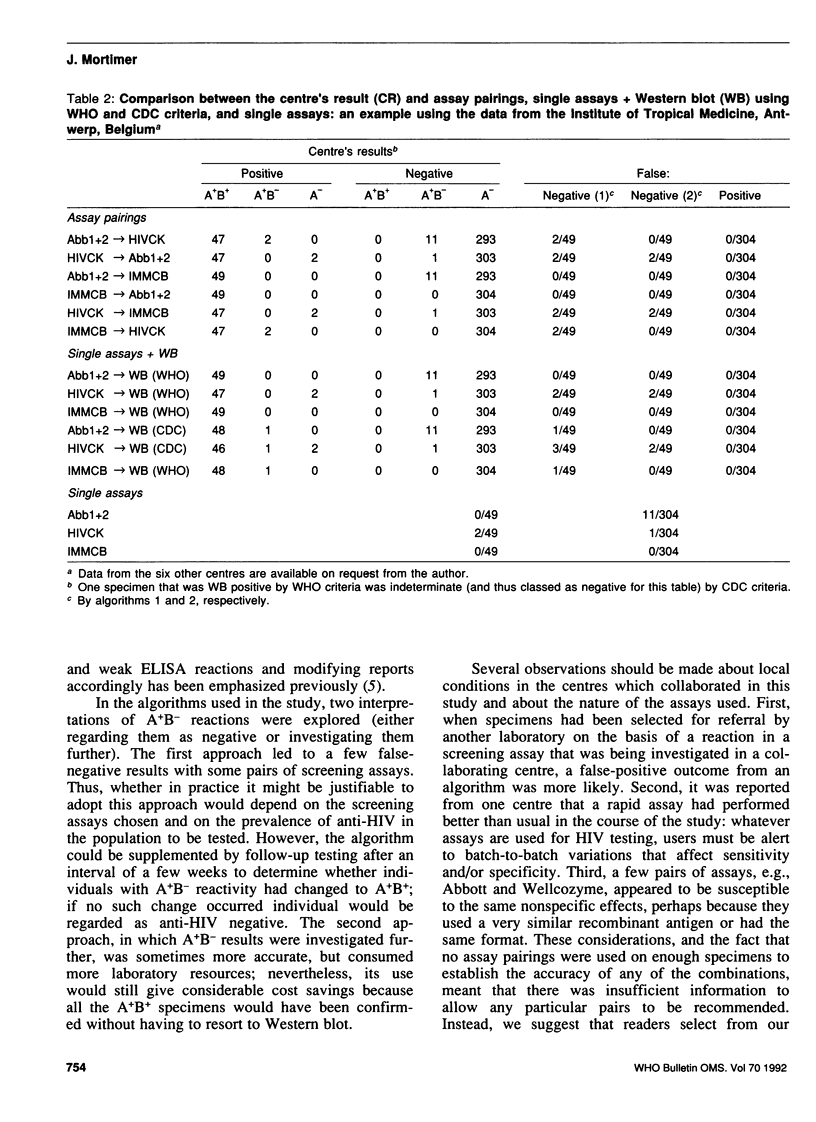
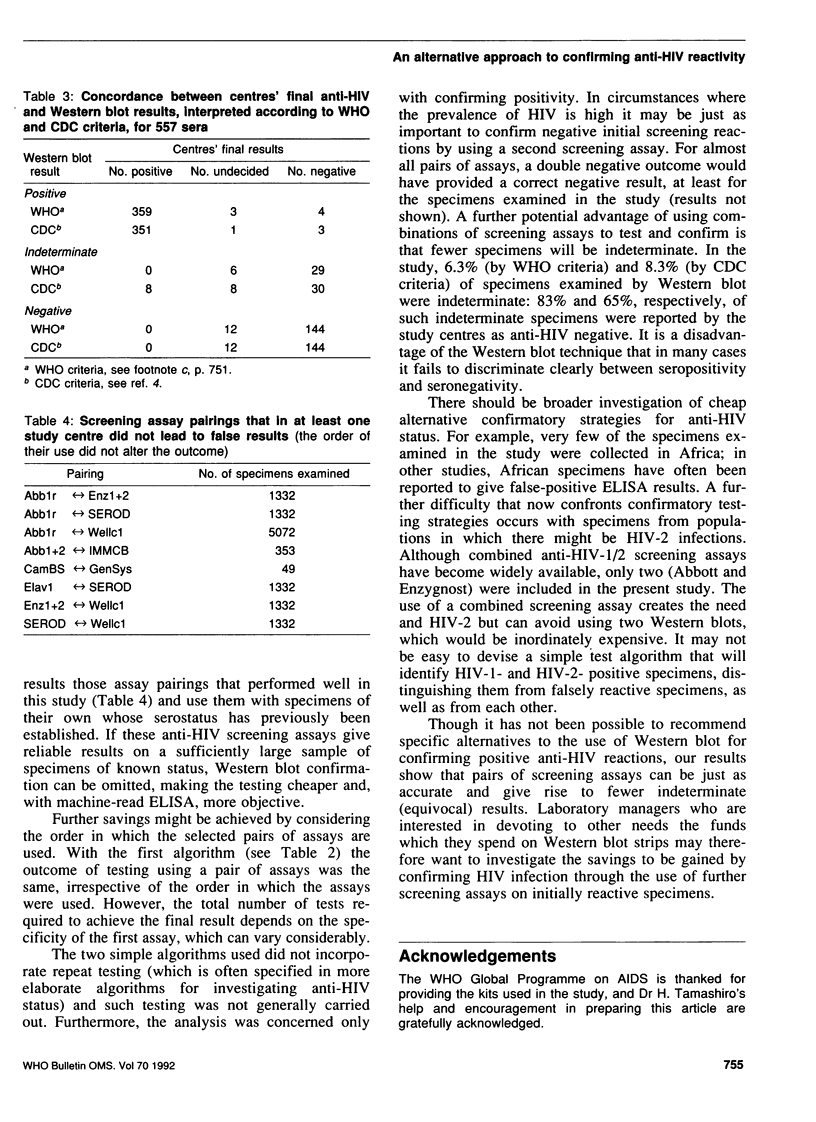
The confirmation of positive screening assay reactions for antibodies to human immunodeficiency virus type 1 (anti-HIV-1) by Western blot is expensive and often gives indeterminate results. We therefore carried out a collaborative study to investigate the confirmation of screening assay reactions using a second screening assay. For this purpose, seven laboratories prospectively tested sequential specimens, using at least one additional screening assay, until about 50 confirmed anti-HIV-1-positive specimens had been identified in each test centre. The reactions of 16 assays were analysed in pairs (assay A and assay B), using assay B on specimens reactive in assay A: A+/B+ reactions were considered positive and A-, negative anti-HIV results. These outcomes were compared with those obtained using confirmatory Western blot. In all, 7950 specimens were tested, and 359 were reported as positive by the laboratories. Within the test centres, eight screening assay pairings gave rise to no false-positive or false-negative results, and these combinations were at least as accurate as a single screening assay followed by Western blot. From 6.3% to 8.3% of the Western blot results were indeterminate. The number of specimens examined was too small to justify recommending for general use named pairs of screening assays; the choice of these would, in any case, depend on local conditions. However, individual laboratory managers may wish to investigate the large potential savings to be made by confirming HIV infection using a second screening assay on initially reactive specimens. If the more sensitive screening assay is used first, the sensitivity of this approach may be improved by further investigation of specimens that react as A+B-.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crofts N., Maskill W., Gust I. D. Evaluation of enzyme-linked immunosorbent assays: a method of data analysis. J Virol Methods. 1988 Oct;22(1):51–59. doi: 10.1016/0166-0934(88)90087-0. [DOI] [PubMed] [Google Scholar]
- Lepine D. G., Neumann P. W., Frenette S. L., O'Shaughnessy M. V. Evaluation of a human immunodeficiency virus test algorithm utilizing a recombinant protein enzyme immunoassay. J Clin Microbiol. 1990 Jun;28(6):1169–1171. doi: 10.1128/jcm.28.6.1169-1171.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg F. A., Kabeya C. M., Quinn T. C., Ryder R. W., Kifuani N. K., Harris J., Bender T. R., Heyward W. L., Tam M. R., Auditore-Hargreaves K. Performance and cost-effectiveness of a dual rapid assay system for screening and confirmation of human immunodeficiency virus type 1 seropositivity. J Clin Microbiol. 1990 Feb;28(2):303–306. doi: 10.1128/jcm.28.2.303-306.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg F., Kabeya C. M., Ryder R. W., Kifuani N. K., Harris J., Bender T. R., Heyward W. L., Quinn T. C. Field testing and comparative evaluation of rapid, visually read screening assays for antibody to human immunodeficiency virus. Lancet. 1989 Mar 18;1(8638):580–584. doi: 10.1016/s0140-6736(89)91610-3. [DOI] [PubMed] [Google Scholar]
- van der Groen G., Van Kerckhoven I., Vercauteren G., Piot P. Simplified and less expensive confirmatory HIV testing. Bull World Health Organ. 1991;69(6):747–752. [PMC free article] [PubMed] [Google Scholar]


