Abstract
Biological invasions contribute to global environmental change, but the dynamics and consequences of most invasions are difficult to assess at regional scales. We deployed an airborne remote sensing system that mapped the location and impacts of five highly invasive plant species across 221,875 ha of Hawaiian ecosystems, identifying four distinct ways that these species transform the three-dimensional (3D) structure of native rain forests. In lowland to montane forests, three invasive tree species replace native midcanopy and understory plants, whereas one understory invader excludes native species at the ground level. A fifth invasive nitrogen-fixing tree, in combination with a midcanopy alien tree, replaces native plants at all canopy levels in lowland forests. We conclude that this diverse array of alien plant species, each representing a different growth form or functional type, is changing the fundamental 3D structure of native Hawaiian rain forests. Our work also demonstrates how an airborne mapping strategy can identify and track the spread of certain invasive plant species, determine ecological consequences of their proliferation, and provide detailed geographic information to conservation and management efforts.
Keywords: biological invasion, Hawaii, imaging spectroscopy, LiDAR, tropical forest
The three-dimensional (3D) structure of forest canopies affects the distribution and absorption of solar radiation, the growth and recruitment of plants, and the use of forest resources by insects, birds, and mammals, including humans (1–6). Changes in canopy structure are obvious when forests are planted or cleared, but other structural changes are more difficult to assess. Invasive alien species contribute to environmental change by altering the composition and functioning of ecosystems (7–9), but many studies have documented only the local-scale spread of invasive plants into forests and their local consequences (10–13). The complexity and natural variability of forest ecosystems limit the extent to which local field studies can be used to characterize the regional-scale effects of biological invasions on forest structure and functioning.
Remote sensing provides a synoptic view of ecosystems, but the location of invasive species in forests, much less their ecological consequences, has proven difficult to detect from satellite or aircraft sensors. Traditional aircraft measurements, such as digital photography, often fail to provide the spectral information needed to locate a particular species reliably (14). Satellite instruments are usually too coarse in spatial resolution, spectral information, or both. Resolving both the presence and the ecological impacts of invasive species requires specialized equipment and analytical approaches (15).
We deployed an airborne remote sensing system designed to measure the composition, physiology, and structure of ecosystems. The Carnegie Airborne Observatory (CAO) integrates high-fidelity imaging spectrometers (HiFIS) with light detection and ranging (LiDAR) sensors for regional-scale ecological research (16). The HiFIS subsystem provides detailed canopy spectroscopic reflectance signatures that express plant chemistry, physiological status, and taxonomic composition (17). The LiDAR subsystem provides 3D structural information on canopies, underlying vegetation, and the terrain below [for additional details, see Materials and Methods in the supporting information (SI) Appendix]. The CAO HiFIS and LiDAR are physically and digitally coaligned and packaged with a high-performance inertial navigation system that provides highly accurate determinations of aircraft position and the location of ground targets in three dimensions. Here, we report the use of an approach to locate invasive alien plants and to quantify their impacts on the 3D structure of Hawaiian rain forests.
Results and Discussion
Our results were compiled from 221,875 ha of lowland, submontane, and montane Hawaiian forests aerially surveyed in five regions spanning elevations from 3 to 1,550 m (Fig. 4 in the SI Appendix). The HiFIS, LiDAR, and inertial navigation data were processed together to identify native and invasive species based on their often unique spectral and structural properties, as illustrated in Fig. 1 and described methodologically in Materials and Methods in the SI Appendix. The same aircraft data were then used to develop maps of canopy height and 3D structure by using data fusion algorithms (Figs. 5 and 6 and Materials and Methods in the SI Appendix).
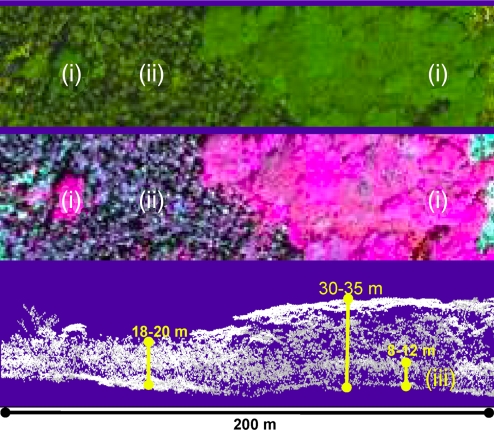
Fig. 1.
Example of high-resolution 3D imagery from the CAO collected over a sample hectare of lowland rain forest on Hawaii Island. (Top) Natural color imagery of invasive F. moluccana (i) and native canopies (ii). (Middle) Hyperspectral mapping of native (ii; blues-greens) and invasive (i; pinks-reds) canopies. (Bottom) Sectional view of the 3D architecture of the canopies, revealing the presence, vertical layering, and overall height of a midlevel canopy (iii) dominated by dense stands of highly invasive P. cattleianum trees. Typical range of tree heights is shown in yellow.
Field validation tests yielded <7% error in the spectroscopic detection of invasive canopy tree species (Table 2 and Materials and Methods in the SI Appendix). Detections at the understory level were 4–6% uncertain (Table 3 and Materials and Methods in the SI Appendix), indicating the efficacy of the combined HiFIS and LiDAR data for penetrating the rain forest canopy. Errors for canopy heights ranged from 0.5 to 0.9 m. Errors caused by colocating the field and remote sensing data were apportioned mostly to the field-based global positioning system (GPS) units (≤4.5 m error), whereas the aircraft and image position uncertainty were both <0.5 m (Materials and Methods in the SI Appendix).
We compiled remote sensing information for 10 focal areas of 49–97 ha each; these areas consisted of five pairs of native-dominated and neighboring alien-dominated forest canopies detected from the air. We also controlled for elevation, substrate age, and land use (see Materials and Methods in the SI Appendix). The tree species Metrosideros polymorpha was found to dominate the native stands, with many other native trees of secondary importance scattered throughout the forests (18, 19). In the invaded forest areas, we mapped the location of the following highly invasive species: Falcataria moluccana, Fraxinus uhdei, Hedychium gardnerianum, Morella faya, and Psidium cattleianum (see Materials and Methods in the SI Appendix). Stand-level statistics were compiled for each species detected to quantify differences in the 3D structure of forests (Fig. 2). Using a comparative 3D analysis between paired native-invaded stands, we uncovered four distinct patterns of forest structural change resulting from different invaders.
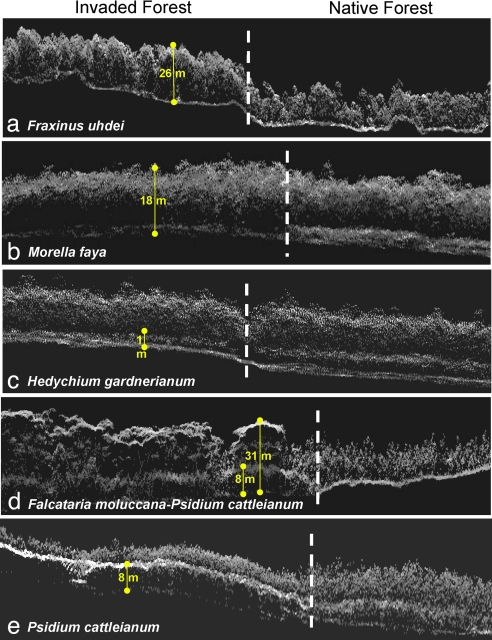
Fig. 2.
Sectional views of adjacent forest stands dominated by either invasive (Left) or native (Right) forests. Each panel shows a dominant invader on the Left: (a) F. uhdei in montane moist forest; (b and c) M. faya and H. gardnerianum in submontane rain forests; (d) F. moluccana and P. cattleianum in lowland rain forest; (e) P. cattleianum alone in lowland rain forest. Mean vegetation heights of invaders are shown in yellow.
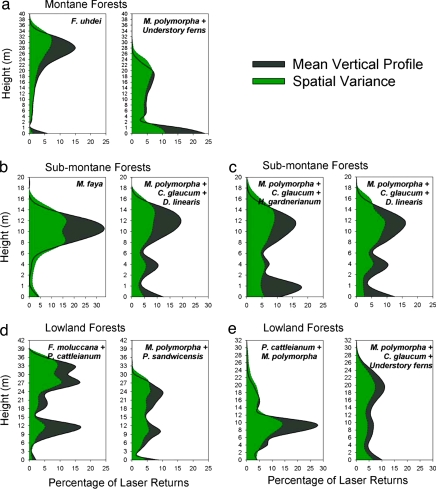
In submontane to montane rain forests, the invasive tree species F. uhdei (Mauna Kea Volcano) and M. faya (Kilauea Volcano) have reached heights of 33 ± 3 m and 17 ± 2 m, respectively (Fig. 8 and Materials and Methods in the SI Appendix). Vertical profiles accumulated over 312 ha of individual tree canopies indicated that F. uhdei and M. faya maintain 32–51% greater biomass volume in the upper canopy than do native species (Fig. 3 a and b), decreasing available light to the understory (Table 4 in the SI Appendix), resulting in almost no species at lower levels in the forest (Table 1). Field measurements guided by the remotely sensed data confirmed that only 2–4% of incoming light penetrates the canopies of mature F. uhdei or M. faya vs. 9–13% light penetration in adjacent native forests (Table 4 in the SI Appendix). We also identified the midcanopy species potentially missing from invaded sites, which included native tree ferns (e.g., Cibotium glaucum) and smaller native woody species such as Cheirodendron trigynum and Ilex anomala. The understory of these invaded forests contained few native ground-covering ferns such as Dicranopteris linearis or native seedlings, as were usually present in their native counterpart forests (20). In sum, these tall, high-leaf-volume invaders create a biologically impoverished environment beneath their canopies (Fig. 10 in the SI Appendix).
Fig. 3.
Statistics of canopy vertical profiling results compiled over large forest tracts (Table 1) dominated by neighboring native or invasive trees as labeled. The vertical profiles quantify the vegetation volume by the percentage of total laser hits in 1-m vertical partitions throughout the canopies.
Table 1.
Stand-level comparisons of 3D vegetation density between native- and invasive-dominated forests
| Forest stand | Cumulative area, ha | Canopy | Midlevel | Understory |
|---|---|---|---|---|
| Montane forest | ||||
| F. uhdei | 60.2 | 81.9 ± 5.4* | 8.3 ± 0.5* | 5.9 ± 2.7* |
| Native | 60.7 | 62.4 ± 3.4† | 26.5 ± 1.9† | 11.4 ± 1.6† |
| Submontane forest | ||||
| M. faya | 97.2 | 95.2 ± 13.1* | — | 4.8 ± 2.2* |
| Native | 94.3 | 62.5 ± 7.6† | 25.0 ± 2.8 | 12.5 ± 6.0† |
| Submontane forest | ||||
| H. gardnerianum | 61.1 | 51.9 ± 6.5* | 9.6 ± 1.1* | 38.5 ± 5.0* |
| Native | 63.8 | 60.5 ± 5.8* | 27.6 ± 2.7† | 11.9 ± 4.0† |
| Lowland forest | ||||
| F. moluccana | 51.9 | 78.5 ± 4.9* | 13.0 ± 0.5* | 8.5 ± 0.8* |
| Native | 48.8 | 50.5 ± 3.8† | 37.6 ± 2.7† | 11.9 ± 3.0† |
| Lowland forest | ||||
| P. cattleianum | 79.7 | 17.5 ± 2.0* | 78.3 ± 9.3* | 4.2 ± 0.5* |
| Native | 80.8 | 55.0 ± 4.2† | 29.3 ± 0.8† | 10.1 ± 3.0† |
Values are the mean (± SE) percentages of total forest canopy volume defined by the number of airborne laser returns. Symbols indicate differences at each vertical stratum in the forest canopy (t tests, P < 0.05). Dash (—) indicates foliage densities <1%.
A second pattern of invasion occurred in submontane rain forests along the eastern flank of Kilauea Volcano. There, native M. polymorpha forests almost always included a midlevel canopy comprised of native C. glaucum tree ferns and many native woody species and an understory dominated by native ground ferns (Fig. 2c). The upper canopy, midcanopy, and understory strata accounted for 60.5 ± 5.8%, 27.6 ± 2.7%, and 11.9 ± 4.0%, respectively, of the volume of these forests (Fig. 3c). Adjacent forests invaded by the understory herb H. gardnerianum had a 3-fold greater understory volume and ≈66% lower native plant volume in the midcanopy (Table 2 and Fig. 7 in the SI Appendix). Plot-scale studies have shown that H. gardnerianum reproduces vegetatively and by seed, proliferating throughout the forest understory and forming an impenetrable layer of rhizomes (21, 22), which is likely the cause of the losses we mapped among mid- and ground-level native species. Although our regional mapping cannot produce the causal mechanisms for species replacement, other research has already shown that this invader causes significant decreases in nutrient concentrations of overstory trees (15) and in the regeneration of native canopy species (22).
A third type of invasion was mapped across lowland rain forests on the east flank of Kilauea Volcano, where the fast-growing tree F. moluccana has proliferated over large areas of rain forest reserve (11, 23). Our airborne measurements indicated that this species now averages 33 ± 4 m in height (Figs. 2d and 3d), while maintaining a relatively low leaf area index (LAI) that allows an average 21% of the incoming sunlight to pass through its canopy (Table 4 and Fig. 10 in the SI Appendix). Beneath F. moluccana trees, we also detected P. cattleianum, a small tree introduced from Brazil that formed a dense midcanopy layer averaging 10 ± 2 m in height (Fig. 2), with high LAI that decreased light penetration by 95–97% (Table 4 in the SI Appendix). Our airborne measurements showed that this combination of invaders change the 3D volume of the forest by +55%, −66%, and −29% at upper, middle, and lower canopy levels, respectively (Table 1). Previous plot-level work suggested that F. moluccana may facilitate P. cattleianum invasion by increasing nitrogen availability via symbiotic fixation (11). Our remote sensing results strongly suggested that the secondary invasion of P. cattleianum leads to low regeneration of native species in the understory, as illustrated in the mean vertical profiles in Fig. 3d. In the less common case where F. moluccana had invaded without P. cattleianum, we found both native and nonnative understory plants growing in high densities and abundances.
A final invasion scenario was identified in the 3D images where P. cattleianum had reached the upper canopy in some lowland rain forests (Figs. 2e and 3e). Throughout 81 ha of compiled detections (Fig. 9 in the SI Appendix), P. cattleianum formed a densely packed layer of tree crowns 9 ± 3 m in height, with mature M. polymorpha trees protruding very sparsely (<20% cover) to 22 ± 3 m height through the alien canopy. In these forest stands, P. cattleianum nearly tripled the volume of plant material in the midcanopy while decreasing the canopy volume in the overstory and understory by 55% and 60%, respectively (Table 1). Both airborne LiDAR and field measurements of these forests indicated that the invader allows only ≈5% of incoming light to pass into the understory. In this scenario, the invasive tree grows within a native canopy, closing at the midlevel position, and precluding the establishment and regeneration of other species, a phenomenon documented by a recent plot-scale study in lowland Hawaiian rain forest (24).
Our work quantifies that a diverse array of alien plant species, each representing a different growth form (e.g., tall tree, short tree, or herb) and/or functional type (e.g., nitrogen-fixing), is fundamentally changing the 3D structure of Hawaiian forests. Our findings are spatially robust in that they integrate structural and functional patterns across hundreds of hectares (Table 1). Previous field studies have demonstrated the cascading local-scale effects of forest compositional changes on the flow of nutrients in these systems (11, 12). However, results from past research are difficult to expand beyond small study areas, given the spatial variability of forest landscapes and the variable nature of the invasion process. Our airborne measurements encompass the heterogeneity of broad landscapes, indicating that a regional-scale process of major ecological change is well under way.
We cannot be sure that invasive plant dominance causes native canopy losses in all of our sites. In some areas, alien plants may fill otherwise unoccupied canopy space. They also spread with forest disturbance: treefall events, storm damage, stand dieback, and feral pigs are widely implicated in the conversion of native rain forest canopies to those now dominated by invasive species (25). However, most invasions detected were observed as large pockets of alien species that stretched outward from a densely populated nucleus or in large stretches of nonnative canopies appearing as invasion fronts (Figs. 7–9 and Materials and Methods in the SI Appendix), and the structural changes that we mapped were coincident with dominance by aliens. These patterns suggest a spread of species through the forests even without the direct aid of disturbance. Independent of the causal mechanisms, our results indicate that four types of invasion scenarios lead to fundamental structural changes to native Hawaiian rain forests, likely with long-lasting impacts on biological diversity and ecosystem function.
All of our invasive species detections were made in protected state and federal rain forest reserves. The ability of these and other alien species to spread across protected areas, often unaided by changes in land use or other human activities (25–27), suggests that traditional rain forest conservation approaches such as ground surveys and localized plant removal, fencing, and enforcement are insufficient to ensure the long-term survival of Hawaii's remaining native rain forest ecosystems. Efforts to control invasive species have a relatively long history in Hawaii, with mixed success (28, 29). Our work demonstrates how airborne technologies, which simultaneously probe the spectral and structural properties of rain forest canopies, can determine changes in forest composition resulting from invasion, thereby contributing to the detection of invasions at early stages and to the analysis of their ecological effects on a regional basis. This capability can make regional conservation planning and action for the maintenance of native biodiversity over large forested areas more tractable than in the past.
Materials and Methods
The CAO was developed for airborne mapping of the biochemical, taxonomic, and structural properties of vegetation and ecosystems (16). The CAO integrates three subsystems into a single sensor package: HiFIS, LiDAR scanner, and GPS–inertial measurement unit (GPS-IMU). The CAO HiFIS subsystem provides spectroscopic images of the land surface; each image pixel contains numerous spectral bands covering either the 367- to 1,058-nm or 380- to 2,510-nm range, depending on system configuration. The LiDAR subsystem provides 3D information on vegetation structure, including height, architecture, and canopy density (30). The GPS-IMU subsystem provides 3D positioning and attitude data for the sensor package onboard the aircraft, allowing for highly precise and accurate projection of HiFIS and LiDAR observations on the ground. Detailed information on the CAO hardware and algorithms are available in Materials and Methods in the SI Appendix.
From January through February 2007, we mapped 221,875 ha covering portions of federal and state forest reserves and parks including Hawaii Volcanoes National Park, Laupahoehoe Forest Reserve, Wao Kele O Puna Reserve, Kohala Forest Reserve, and the Lower Puna reserves of Keauohana, Malama Ki, and Nanawale (Fig. 4 in the SI Appendix). The data were collected from 1 to 2.5 km altitude above ground level, providing mapping results at a spatial resolution from 0.5 to 2.5 m. The spectral and LiDAR data were combined to detect five invasive species as described in Materials and Methods in the SI Appendix. These detections were then aggregated by species and compared locally with paired stands of predominantly native species. To quantify differences in forest volumes among comparative stands, we calculated changes in LiDAR point densities among canopy strata (e.g., lower-, mid-, and upper-canopy positions).
Our field studies were designed to evaluate the accuracy of our remote species detections across large tracts of rain forest. To do so, we used a combination of intensive plot-scale measurements, long field-based transect surveys, and low-altitude helicopter surveys, all guided by the remotely sensed data, to identify false-positive and false-negative detections. Transects ranged from 200 m to 3,000 m in length, with a total distance covered among sites of 16.3 km (see Materials and Methods in the SI Appendix). We also collected LAI measurements in each forest by using a plant canopy analyzer (LAI-2000; Licor). These measurements included an estimate of mean leaf angle, which, when combined with LAI, provided a means to estimate the fractional absorption of photosynthetically active radiation by each canopy with a radiative transfer model. These data were used to quantify and validate further the impacts of the invasive species on canopy structure and the light environment.
Supplementary Material
Acknowledgments.
The Carnegie Airborne Observatory is made possible by the support of the W. M. Keck Foundation and William Hearst III. This application of the Carnegie Airborne Observatory was supported by NASA Terrestrial Ecology Program Biodiversity Grant NNG-06-GI-87G. Access to field sites was provided by the U.S. National Park Service and the State of Hawaii Department of Land and Natural Resources, Division of Forestry and Wildlife.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710811105/DC1.
References
- 1.Smith AP, Hogan KP, Idol JR. Biotropica. 1992;24:503–511. [Google Scholar]
- 2.Brokaw NV, Scheiner SM. Ecology. 1989;70:538–541. [Google Scholar]
- 3.Clark DB, Read JM, Clark M, Cruz AM, Dotti MF, Clark DA. Ecol Appl. 2004;14:61–74. [Google Scholar]
- 4.Cummings DL, Kauffman JB, Perry DA, Hughes RF. Forest Ecol Manage. 2002;163:293–307. [Google Scholar]
- 5.Denslow JS. Annu Rev Ecol Syst. 1987;18:431–451. [Google Scholar]
- 6.Leigh EG. Annu Rev Ecol Syst. 1975;6:67–86. [Google Scholar]
- 7.D'Antonio CM, Vitousek PM. Annu Rev Ecol Syst. 1992;23:63–87. [Google Scholar]
- 8.Vitousek PM, D'Antonio CM, Loope LL, Rejmanek M, Westbrooks R. New Z J Ecol. 1997;21:1–16. [Google Scholar]
- 9.Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. Science. 1997;277:494–499. [Google Scholar]
- 10.Huenneke LF, Vitousek PM. Biol Conserv. 1990;53:199–211. [Google Scholar]
- 11.Hughes FR, Uowolo A. Ecosystems. 2006;9:977–991. [Google Scholar]
- 12.Vitousek PM, Walker LR. Ecol Monogr. 1989;59:247–265. [Google Scholar]
- 13.Rejmanek M, Richardson DM, Higgins SI, Pitcairn MJ, Grotkopp E. In: Invasive Alien Species: A New Synthesis. Mooney HA, Mack RN, McNeely JK, Neville LE, Schei PJ, Waage JK, editors. Washington, DC: Island; 2005. pp. 104–161. Vol SCOPE 63. [Google Scholar]
- 14.Mack RN. In: Invasive Alien Species: A New Synthesis. Mooney HA, Mack RN, McNeely JK, Neville LE, Schei PJ, Waage JK, editors. Washington, DC: Island; 2005. pp. 179–208. Vol. SCOPE 63. [Google Scholar]
- 15.Asner GP, Vitousek PM. Proc Natl Acad Sci USA. 2005;102:4383–4386. doi: 10.1073/pnas.0500823102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asner GP, Knapp DE, Kennedy-Bowdoin T, Jones MO, Martin RE, Boardman J, Field CB. J Appl Remote Sens. 2007 doi: 10.1117/1.2794018. [DOI] [Google Scholar]
- 17.Ustin SL, Roberts DA, Gamon JA, Asner GP, Green RO. Bioscience. 2004;54:523–534. [Google Scholar]
- 18.Wagner WL, Herbst DR, Sohmer SH. Manual of the Flowering Plants of Hawai'i. Honolulu: Univ Hawaii Press and Bishop Museum; 1999. [Google Scholar]
- 19.Stemmermann L. Pac Sci. 1983;37:361–373. [Google Scholar]
- 20.Walker LR, Aplet GH. Biotropica. 1994;26:378–383. [Google Scholar]
- 21.Anderson RC, Gardner DE. Biol Control. 1999;15:89–96. [Google Scholar]
- 22.Williams PA, Winks C, Rijkse W. New Z J Ecol. 2003;27:45–54. [Google Scholar]
- 23.Hughes FR, Denslow JS. Ecol Appl. 2005;15:1615–1628. [Google Scholar]
- 24.Zimmerman N, Hughes RF, Cordell S, Hart P, Chang HK, Perez D, Like RK, Ostertag R. Biotropica. 2007 doi: 10.1111/j.1744-7429.2007.00371.x. [DOI] [Google Scholar]
- 25.Smith CW. In: Hawaii's Terrestrial Ecosystems: Preservation and Management. Stone CP, Scott JM, editors. Honolulu: Cooperative National Park Resources Study Unit, Univ of Hawaii at Manoa; 1985. pp. 180–250. [Google Scholar]
- 26.Loope LL, Mueller-Dombois D. In: Biological Invasions: A Global Perspective. Drake J, DiCastri F, Groves R, Kruger F, Mooney HA, Rejmanek M, Williamson M, editors. Chichester, UK: Wiley; 1989. pp. 257–280. [Google Scholar]
- 27.Vitousek PM. In: Ecology of Biological Invasions of North America and Hawaii. Mooney HA, Drake JA, editors. New York: Springer; 1986. pp. 163–176. Vol Ecological Studies. [Google Scholar]
- 28.Gardner DE, Davis CJ. The Prospects for Biological Control of Nonnative Plants in Hawaiian National Parks. Honolulu: Cooperative National Park Study Unit, Univ of Hawaii at Manoa, Dept of Botany; 1982. Tech Rept 45. [Google Scholar]
- 29.Cabin RJ, Weller SG, Lorence DH, Cordell S, Hadway LJ, Montgomery R, Goo D, Urakami A. Ecol Appl. 2002;12:1595–1610. [Google Scholar]
- 30.Lefsky MA, Cohen WB, Parker GG, Harding DJ. BioScience. 2002;52:19–30. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.