Abstract
Rhabdomyosarcoma (RMS) is a common paediatric soft tissue sarcoma that resembles developing foetal skeletal muscle. Tumours of the alveolar subtype frequently harbour one of two characteristic translocations that juxtapose PAX3 or PAX7, and the forkhead-related gene FKHR (FOXO1A). The embryonal subtype of RMS is not generally associated with these fusion genes. Here, we have quantified the relative levels of chimaeric and wild-type PAX transcripts in various subtypes of RMS (n=34) in order to assess the relevance of wild-type PAX3 and PAX7 gene expression in these tumours. We found that upregulation of wild-type PAX3 is independent of the presence of either fusion gene and is unlikely to contribute to tumorigenesis. Most strikingly, upregulated PAX7 expression is almost entirely restricted to cases without PAX3-FKHR or PAX7-FKHR fusion genes and may contribute to tumorigenesis in the absence of chimaeric PAX transcription factors. Furthermore, as myogenic satellite cells are known to express PAX7, this pattern of PAX7 expression suggests this cell type as the origin of these tumours. This is corroborated by the detection of MET (c-met) expression, a marker for the myogenic satellite cell lineage, in all RMS samples expressing wild-type PAX7.
Keywords: rhabdomyosarcoma, PAX3, PAX7, FOXO1A, myogenesis, MET
Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma of childhood, accounting for up to 8% of all cases of childhood cancer (Cornelison and Wold, 1997). This embryonal muscle cancer resembles normal preinnervated foetal skeletal muscle, both morphologically and in its expression of muscle-specific genes (including the muscle regulatory factor (MRF) family and skeletal muscle-specific structural proteins). There are distinct histological subtypes of RMS that differ in their clinical presentation and behaviour: the embryonal subtype (ERMS) is more common in young children and has a better prognosis, whereas the alveolar subtype (ARMS) is more common in adolescents, is frequently metastatic at diagnosis, and has a worse prognosis. In addition, a rare pleomorphic subtype (PRMS) predominates in adults, among whom RMS is relatively infrequent (Hollowood and Fletcher, 1994).
The histogenesis of RMS is unclear, although it is generally believed to originate from persistent foetal rhabdomyoblasts that have failed to undergo normal differentiation. Studies of expression patterns of myogenic genes have failed to demonstrate any differences in extent of myogenic differentiation between the two main subtypes, ERMS and ARMS. However, comparative genomic hybridisation (CGH) analysis has shown that ERMS frequently exhibits gains or losses of specific whole chromosomes, whereas ARMS is characterised by the presence of regions of genomic amplification (Weber-Hall et al, 1996; Gordon et al, 2001). The finding of consistent reciprocal chromosomal translocations only in ARMS has suggested a biological basis for the difference in tumour aggressiveness. Alveolar tumours typically harbour one of the two characterised chromosomal translocations that juxtapose PAX gene family member PAX3 or PAX7 with the Forkhead-related FKHR (FOXO1A) gene. The t(2;13)(q35;q14) translocation results in the PAX3-FKHR fusion gene, and the less common t(1;13)(p36;q14) produces the PAX7-FKHR fusion gene. Both translocations generate an in-frame fusion between the undisrupted PAX gene DNA-binding domain and the transactivation domain of the FKHR gene.
During normal myogenesis, PAX3 is expressed in migrating myoblasts and is believed to inhibit their differentiation until they reach their destination. It has been suggested that dysregulated expression of PAX3, and/or its normal target genes, is involved in RMS tumorigenesis (Bober et al, 1994; Goulding et al, 1994; Epstein et al, 1996). The chimaeric PAX3-FKHR protein has been studied in vitro and shown to retain wild-type PAX3 DNA-binding specificity, but with enhanced transcriptional activation. PAX3-FKHR is therefore a more powerful transcription factor than wild-type PAX3. (Fredericks et al, 1995; Bennicelli et al, 1996; Anderson et al, 1999; Merlino and Helman, 1999). PAX3-FKHR transforms chicken embryo fibroblasts, although wild-type PAX3 is unable to do so (Schiedler et al, 1996), and PAX3-FKHR inhibits the differentiation of the C2C12 murine myoblastic cell line to a greater extent than wild-type PAX3 (Epstein et al, 1995). This suggests that enhanced PAX3 activity, through increased transcription levels and transcriptional activity of the PAX3-FKHR chimaeric protein, may be contributing to the oncogenic phenotype of RMS. PAX7 expression in normal myogenesis is concurrent with PAX3 expression in myoblasts in the dermomyotome, although PAX7 expression does not persist in migrating myoblasts. It has been shown that the PAX7-FKHR fusion gene is frequently amplified in ARMS, suggesting that increased gene dosage may be important in controlling its altered function (Barr et al, 1996; Weber-Hall et al, 1996; Anderson et al, 1999; Barr, 1999, 2001; Fitzgerald et al, 2000).
PAX3 and PAX7 appear to have a high degree of functional redundancy in normal myogenesis (Mansouri, 1998), although recent work has proposed that expression of PAX7, and not PAX3, is required for the specification and maintenance of myogenic satellite cells, a distinct lineage of myoblastic precursor cells responsible for the postnatal growth, repair and maintenance of skeletal muscle (Seale et al, 2000). Expression of PAX3 and PAX7 has previously been detected in RMS cell lines (Barr et al, 1999), here we have measured levels of expression of the PAX genes and their chimaeric PAX-FKHR derivatives in primary RMS samples. The observations that PAX3-FKHR is a more potent transcription factor than PAX3, and that PAX7-FKHR is frequently amplified in RMS cases, suggest that there may be a ‘dose’ effect of increased PAX3/PAX7 activity in RMS (Ginsberg et al, 1998). Hence, in tumours without PAX3/7-FKHR fusion genes, it is possible that mechanisms other than those associated with PAX3/7-FKHR fusion genes cause increased expression of wild-type PAX genes, with the same tumorigenic outcome as expression of the chimaeric proteins. Additionally, levels of PAX gene expression may reflect the cellular origin and stage of myogenic differentiation associated with the tumour.
Real-time PCR accurately measures the copy number of a specific mRNA species in a small sample of total RNA (<1 μg), and is useful for analysis of gene expression in primary RMS tumours because samples are seldom large enough for protein analysis. We used this technique to measure mRNA levels for wild-type PAX3, PAX7, PAX3-FKHR and PAX7-FKHR in RMS, to determine whether higher levels of wild-type PAX3 and PAX7 are observed in tumours that do not express PAX3-FKHR and PAX7-FKHR chimaeric transcripts. Expression of a putative target gene for upregulation by PAX3 and PAX3-FKHR, the oncogene MET, has also been measured by RT–PCR to determine whether upregulation of wild-type PAX3/7 activity in the absence of PAX3/7-FKHR fusion genes may be having a downstream effect.
METHODS AND MATERIALS
Preparation of cDNA from RMS samples
The RMS samples have been previously characterised and described (Anderson et al, 2001). Total RNA was prepared from frozen RMS primary tumour samples and cell lines using Trizol (Life Technologies, Inc., Scotland, UK) according to the manufacturer's instructions. cDNA was synthesised from 1 μg total RNA using reverse transcriptase ‘Superscript’ (Life Technologies) and random hexamers (Life Technologies). To determine cDNA integrity, PCR was used to detect the ubiquitous housekeeping gene GAPDH (primer 1: 5′-CGGGAAGCTTGTGATCAATGG-3′, primer 2: 5′-GGCAGTGATGGCATGGACTG-3′, Tm=55°C, [Mg2+]=2.0 mM, product size=358 bp, 35 cycles).
Real-time PCR to detect levels of PAX3, PAX7, PAX3-FKHR and PAX7-FKHR mRNA
A multiplex TaqMan® reaction was used to measure levels of the gene of interest, and of 18S rRNA. The gene of interest was detected using a FAM/TAMRA-labelled probe, and the rRNA internal control was detected using a VIC/TAMRA-labelled probe. The reaction was performed in 25 μl of 1 × TaqMan® Universal PCR Mix (Applied Biosystems, Foster City, CA, USA) containing primers, probes and cDNA template. Primers and probes were designed to amplify across the PAX-FKHR breakpoint in the fusion genes, and the equivalent region of the wild-type PAX3 and PAX7 genes, and also to amplify across an exon/exon boundary to ensure no genomic DNA would be amplified. Optimal primer and probe concentrations were determined empirically. Table 1 shows primers and probes used for the reactions. Cycling parameters used were 2 min at 50°C, 10 min at 95°C, and then 15 s at 95°C followed by 1 min at 60°C for 40 cycles. The reactions were carried out on the ABI Prism 7700 DNA sequencer. Relative expression of the gene of interest and 18S rRNA was determined from a standard curve generated from a dilution series of known positive RMS samples. The RD RMS cell line (RDCL) (American Type Cell Culture, Manassas, VA, USA) was used as a positive control for both PAX3 and PAX7 expression, as expression of both the genes was readily detectable in this cell line by conventional RT–PCR (35 cycles). RMS cell line Rh30 was used as a positive control for PAX3-FKHR expression, and in the absence of known RMS cell lines expressing the PAX7-FKHR fusion gene, a primary tumour (sample 6) with a known t(1;13) translocation was used as a positive control for PAX7-FKHR expression. Expression of the fusion genes was readily detectable in these positive controls by RT–PCR (35 cycles). cDNA (0.1 μl) was used in each Real-Time PCR reaction, and level of relative expression for each gene of interest in each sample was measured in four independent experiments. Translocation status is shown for each tumour and had been previously determined by a combination of three methods (cytogenetics, interphase fluorescent in situ hybridisation and RT–PCR) in a previous study characterising this cohort of samples (Anderson et al, 2001).
Table 1. Primers and probes used to detect PAX3, PAX7, PAX3-FKHR and PAX7-FKHR expression by real-time PCR.
| Gene | Sequence | Concentration (nM) |
| PAX3 | Primer 1: 5′-CACCAGGCATGGATTTTCC-3′ | 50 |
| Primer 2: 5′-TTGTCAGGAGTCCCATTACCT-3′ | 50 | |
| Probe: 5′-CACCATTGGCAATGGCCTCTCA-3′ | 50 | |
| PAX7 | Primer 1: 5′-CCACAGCTTCTCCAGCTACTCTG-3′ | 300 |
| Primer 2: 5′-GGGTTGCCCAAGATGCTC-3′ | 300 | |
| Probe: 5′-CCGGTCAGCAACGGCCTGTCT-3′ | 100 | |
| PAX3-FKHR | Primer 1: 5′-AGGCATGGATTTTCCAGCTATA-3′ | 50 |
| Primer 2: 5′-GGGACAGATTATGACGAATTGAATT-3′ | 300 | |
| Probe: CACCATTGGCAATGGCCTCTCA-3′ | 50 | |
| PAX7-FKHR | Primer 1: 5′-TCTGCCTACGGAGCCCG-3′ | 300 |
| Primer 2: 5-GGGACAGATTATGACGAATTGAATT-3′ | 300 | |
| Probe: 5′-CCGGTCAGCAACGGCCTGTCT-3′ | 100 |
Real-time multiplex PCR of 18S rRNA
The TaqMan® ribosomal RNA control reagents (Applied Biosystems) are designed to detect the 18S rRNA gene in a diverse range of eukaryotes. Levels of 18S rRNA were detected in each sample in tandem with detection of the gene of interest, and used as a control for the total amount of cDNA added to the reaction.
RT–PCR to detect MET in RMS samples
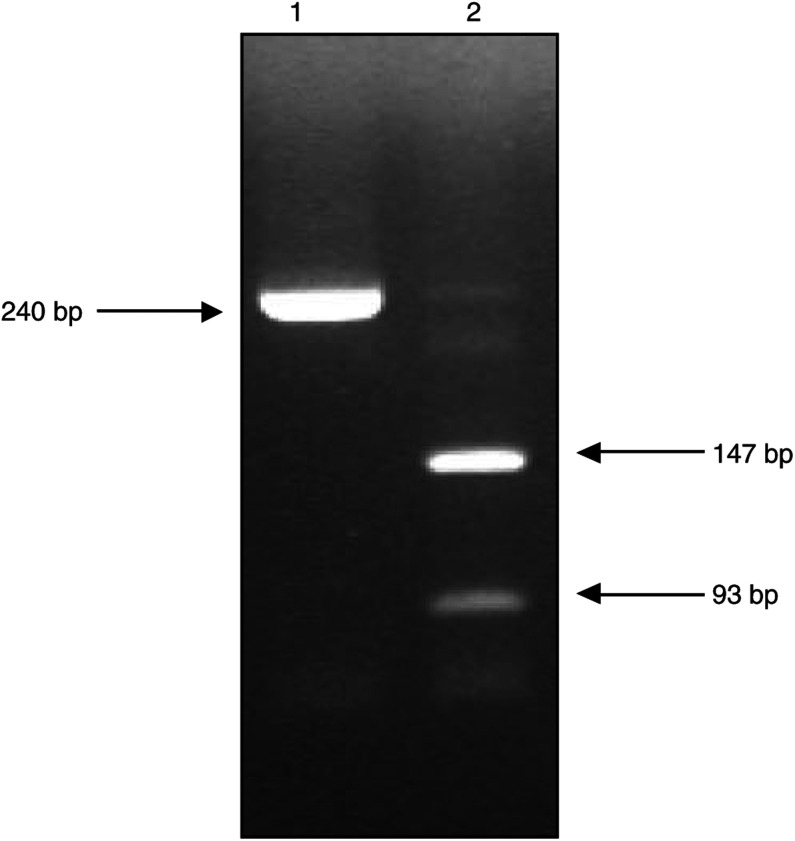
Primers for the MET gene were designed to amplify across an exon/exon boundary to prevent amplification of genomic DNA. The primers were chosen to lie in regions of the sequence not showing significant homology with other known gene family members. Primers used were 5′-TGAATACTGCAGACCAATGTGCTAATAGAT-3′ (forward primer) and 5′-TAGTGATAGATACTGTTCCCTTGTAGCTGC-3′ (reverse primer), (Oswel, University of Southampton, UK), Tm=53°C, 40 cycles. A total volume of 25 μl was used for each PCR (0.5 mM dNTPs, 0.5 μM forward and reverse primers, 2.0 mM Mg, 0.1 μl Taq polymerase and 1.0 μl cDNA). To confirm specificity of the PCR reactions, the amplified cDNA fragment (240 bp) was digested with restriction enzyme HaeIII and DNA fragments (147, 93 bp) were resolved on a 2.5% agarose gel.
RESULTS
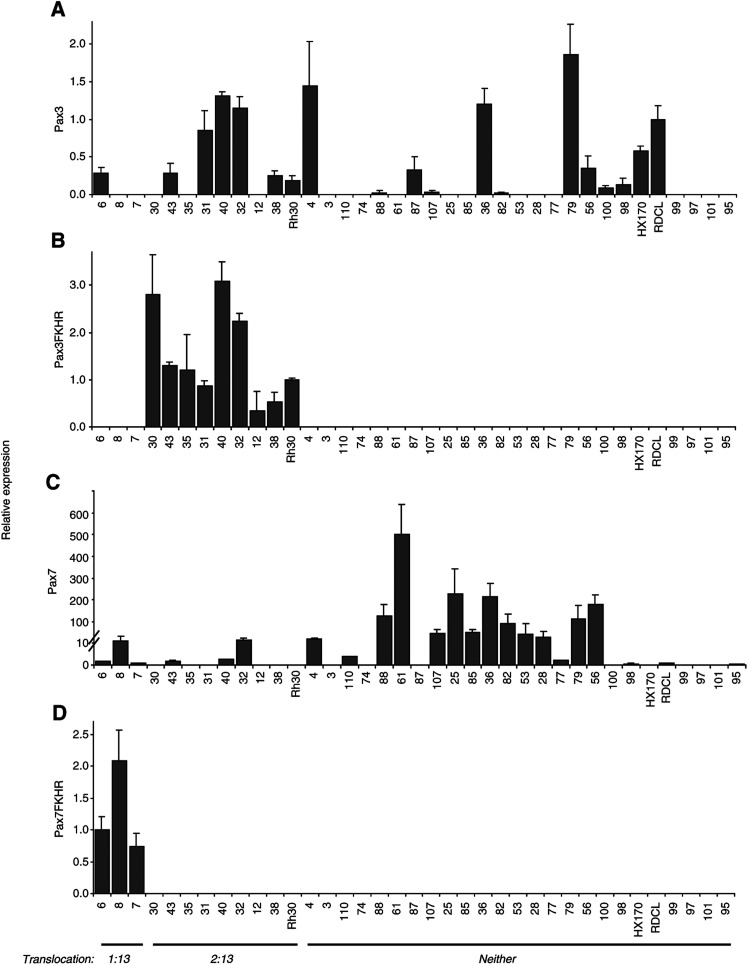
Expression of each of the wild-type PAX3 and PAX7 genes and the two fusion genes PAX3-FKHR and PAX7-FKHR was measured by quantitative real-time PCR in 34 primary RMS tumours and in three RMS cell lines. Expression levels were quantified relative to expression in the RD cell line (RDCL) for PAX3 and PAX7, the Rh30 cell line for PAX3-FKHR and a primary tumour (sample 6) for PAX7-FKHR, since no cell line expressing this fusion gene was available. Table 2 shows the average expression levels determined in four independent experiments. Since our hypothesis is that the mechanism of enhanced PAX3 and/or PAX7 activity will vary according to whether the tumour contains a PAX3/7-FKHR fusion gene, tumours are grouped by translocation status rather than histological subtype. To give an overview of how the levels of expression of the four genes relate to each other, relative PAX3 levels (Figure 1A) are compared to relative PAX3-FKHR levels (Figure 1B), relative PAX7 levels (Figure 1C) and relative PAX7-FKHR levels (Figure 1D).
Table 2. Real-time data for PAX3, PAX3-FKHR, PAX7 and PAX7-FKHR.
|
Pax3 |
Pax3FKHR |
Pax7 |
Pax7FKHR |
|||||||
| Sample I.D. | Trans | Diagnosis | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. |
| 6 | 1 : 13 | ARMS | 0.28 | 0.08 | 0.00 | 0.00 | 1.61 | 0.90 | 1.00 | 0.20 |
| 8 | 1 : 13 | ARMS | 0.00 | 0.00 | 0.00 | 0.00 | 11.90 | 19.96 | 2.08 | 0.48 |
| 7 | 1 : 13 | ARMS | 0.00 | 0.00 | 0.00 | 0.00 | 0.84 | 0.75 | 0.75 | 0.20 |
| 30 | 2 : 13 | ARMS | 0.00 | 0.00 | 2.80 | 0.86 | 0.00 | 0.00 | 0.00 | 0.00 |
| 43 | 2 : 13 | ARMS | 0.28 | 0.13 | 1.29 | 0.08 | 1.84 | 1.51 | 0.00 | 0.00 |
| 35 | 2 : 13 | ARMS | 0.00 | 0.00 | 1.20 | 0.75 | 0.00 | 0.00 | 0.00 | 0.00 |
| 31 | 2 : 13 | ARMS | 0.85 | 0.27 | 0.87 | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 |
| 40 | 2 : 13 | ARMS | 1.32 | 0.05 | 3.08 | 0.41 | 2.43 | 1.10 | 0.00 | 0.00 |
| 32 | 2 : 13 | ARMS | 1.15 | 0.15 | 2.23 | 0.18 | 15.67 | 8.63 | 0.00 | 0.00 |
| 12 | 2 : 13 | ERMS | 0.00 | 0.00 | 0.34 | 0.42 | 0.00 | 0.00 | 0.00 | 0.00 |
| 38 | 2 : 13 | RMS-nos | 0.25 | 0.07 | 0.52 | 0.22 | 0.00 | 0.00 | 0.00 | 0.00 |
| Rh30 | 2 : 13 | Cell Line | 0.18 | 0.07 | 1.00 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 |
| 4 | Neither | RMS-nos | 1.45 | 0.58 | 0.00 | 0.00 | 18.04 | 7.92 | 0.00 | 0.00 |
| 3 | Neither | ARMS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 110 | Neither | ARMS | 0.00 | 0.00 | 0.00 | 0.00 | 3.80 | 2.51 | 0.00 | 0.00 |
| 74 | Neither | ARMS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 88 | Neither | ARMS | 0.02 | 0.03 | 0.00 | 0.00 | 126.71 | 51.76 | 0.00 | 0.00 |
| 61 | Neither | ARMS | 0.00 | 0.00 | 0.00 | 0.00 | 501.39 | 138.76 | 0.00 | 0.00 |
| 87 | Neither | ARMS | 0.33 | 0.17 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 107 | Neither | ARMS | 0.03 | 0.02 | 0.00 | 0.00 | 45.57 | 17.14 | 0.00 | 0.00 |
| 25 | Neither | ERMS | 0.00 | 0.00 | 0.00 | 0.00 | 228.49 | 111.33 | 0.00 | 0.00 |
| 85 | Neither | ERMS | 0.00 | 0.00 | 0.00 | 0.00 | 51.02 | 14.35 | 0.00 | 0.00 |
| 36 | Neither | ERMS | 1.20 | 0.22 | 0.00 | 0.00 | 211.67 | 62.47 | 0.00 | 0.00 |
| 82 | Neither | ERMS | 0.02 | 0.02 | 0.00 | 0.00 | 88.59 | 45.35 | 0.00 | 0.00 |
| 53 | Neither | ERMS | 0.00 | 0.00 | 0.00 | 0.00 | 40.22 | 50.43 | 0.00 | 0.00 |
| 28 | Neither | ERMS | 0.00 | 0.00 | 0.00 | 0.00 | 29.59 | 27.12 | 0.00 | 0.00 |
| 77 | Neither | ERMS | 0.00 | 0.00 | 0.00 | 0.00 | 2.06 | 1.67 | 0.00 | 0.00 |
| 79 | Neither | ERMS | 1.87 | 0.40 | 0.00 | 0.00 | 110.51 | 64.14 | 0.00 | 0.00 |
| 56 | Neither | ERMS | 0.35 | 0.17 | 0.00 | 0.00 | 179.96 | 44.47 | 0.00 | 0.00 |
| 100 | Neither | ERMS | 0.08 | 0.03 | 0.00 | 0.00 | 0.04 | 0.04 | 0.00 | 0.00 |
| 98 | Neither | ERMS | 0.13 | 0.08 | 0.00 | 0.00 | 0.65 | 0.67 | 0.00 | 0.00 |
| HX170 | Neither | Cell Line | 0.58 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| RDCL | Neither | Cell Line | 1.00 | 0.18 | 0.00 | 0.00 | 1.00 | 0.24 | 0.00 | 0.00 |
| 99 | Neither | PRMS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 97 | Neither | PRMS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 101 | Neither | PRMS | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 95 | Neither | PRMS | 0.00 | 0.00 | 0.00 | 0.00 | 0.33 | 0.47 | 0.00 | 0.00 |
Mean values and standard deviations (s.d.) are shown for each gene and each sample. Translocation status (‘Trans’) and histological diagnosis are shown. ARMS=alveolar RMS, ERMS=embryonal RMS, PRMS=pleomorphic RMS, RMS-nos=RMS not otherwise specified. Positive values are shaded.
Figure 1.
Average relative expression of PAX3, PAX3-FKHR, PAX7 and PAX7-FKHR in RMS samples. (A) Expression of PAX3 is shown relative to the expression of PAX3 in the cell line RDCL. (B) Expression of PAX3-FKHR is shown relative to the expression of PAX3-FKHR in the Rh30 cell line. (C) Expression of PAX7 is shown relative to the expression of PAX7 in the cell line RDCL (note different scale due to high levels of expression relative to RDCL=1.0). (D) Expression of PAX7-FKHR is shown relative to the expression of PAX7-FKHR in the RMS sample 6. Translocation status is shown. Error bars indicate standard deviation of four experiments.
Among the ERMS and ARMS, there was no clear pattern for wild-type PAX3 mRNA expression, detected in 16 out of 30 primary ERMS and ARMS tumour samples, nor was there any correlation with the presence of PAX3/7-FKHR fusion genes (Figure 1A, B and D). By contrast, PAX7 mRNA expression corresponded to translocation status rather than histological subtype, with PAX7 expression detected in both ARMS and ERMS, but mainly restricted to tumours lacking either a PAX3-FKHR or a PAX7-FKHR fusion gene. PAX7 expression was detected at lower levels in six out of 11 cases of RMS with PAX3/7-FKHR fusion genes, whereas 15 out of 19 ARMS and ERMS cases lacking the fusion genes expressed PAX7, generally at much higher levels (Table 2 and Figure 1C). Expression of PAX7 was readily detectable by real-time PCR and conventional RT–PCR in the control cell line RDCL (no known translocation); however, levels of PAX7 detected in many of the RMS samples with no known translocation were up to 500 times greater than that of the control cell line (Table 2 and Figure 1C). Adult-type PRMS expressed neither PAX3 nor PAX7.
As expected, expression of the fusion genes was restricted to tumours known to harbour the relevant translocation, with relative expression levels of PAX3-FKHR varying between 0.3- and 3.1-fold and those for PAX7-FKHR between 0.8- and 2.1-fold. Expression of MET was detected in 32 out of 37 RMS samples by RT–PCR (Figure 2). All of the 19 RMS samples with no known translocation and all four of the PRMS tumours were found to express MET mRNA. All three of the tumours with PAX7-FKHR fusion genes expressed MET mRNA, whereas this was not detected in five out of nine samples with the PAX3-FKHR fusion.
Figure 2.
RT–PCR to detect MET. Example of MET PCR product (240 bp) in lane 1, and restriction enzyme digest products (147, 93 bp) in lane 2.
DISCUSSION
We have investigated whether RMS samples without PAX-FKHR fusion genes may have elevated levels of wild-type PAX3 or PAX7 expression, which might be implicated in tumorigenesis or indicate cellular origin of the tumour, and have found that elevated PAX3 expression levels are independent of RMS subtype or presence of a PAX3/7-FKHR fusion gene. PAX3 expression was detected in 16 out of 34 tumour samples, regardless of subtype or translocation status, and it seems unlikely that a ‘dose’ effect of PAX3 activity may confer a transformed phenotype in RMS in which the PAX-FKHR fusion genes do not occur. However, elevated levels of wild-type PAX7 expression are confined mainly to RMS samples with no known translocation (excluding PRMS cases). PAX7 and PAX3 expression levels in these RMS samples are measured relative to expression levels in the RD cell line, which expresses PAX7 levels comparable to those of normal human myoblasts (Bernasconi et al, 1996).
These results agree with data showing PAX3 and PAX7 expression in ERMS cell lines published by Barr et al (1999) and data from RMS specimens referred to therein. Elevated PAX7 expression in ERMS cell lines is also reported by Bernasconi et al (1996). Here we show greatly elevated levels of PAX7 expression in a large cohort of primary ERMS tumours, and generally lower, less frequent expression of PAX7 in primary RMS tumours with PAX-FKHR fusion genes. It is possible in ERMS that elevated wild-type PAX7 expression contributes to transformation in the absence of PAX3-FKHR or PAX7-FKHR expression, perhaps by suppressing the apoptotic programme that would normally eliminate these cells (Bernasconi et al, 1996). PAX3, PAX7 and their chimaeric derivatives were not detected in any of the four PRMS samples included in this study, suggesting that this subtype of RMS is distinct from ERMS and ARMS. This is supported by the distinct histopathology of these tumours and their prevalence in adult rather than paediatric patients (Hollowood and Fletcher, 1994).
PAX7 expression during normal myogenesis is concurrent with PAX3 expression; both genes appear to have the same DNA-binding specificities and a high degree of functional redundancy (Maulbecker and Gruss, 1993; Borycki et al, 1999). However, elevated PAX7 expression in ERMS is not accompanied by elevated PAX3 expression, suggesting that this tumour type is not derived from proliferating myoblasts in the dermomyotome. Recent work has shown that expression of PAX7 rather than PAX3 is required for specification and maintenance of myogenic satellite cells (Seale et al, 2000). These are a distinct lineage of myoblastic precursor cells responsible for the postnatal growth, repair and maintenance of skeletal muscle (Gussoni et al, 1999; Jackson et al, 1999; Seale and Rudnicki, 2000). Analysis of PAX7−/− transgenic mice implicates PAX7 expression in the specification of myogenic satellite cells from uncommitted side population (SP) stem cell progenitors in skeletal muscle, where PAX7 expression may induce satellite cell specification by restricting alternate developmental programmes (Seale et al, 2000). PAX7 expression is an early event during embryogenesis, and satellite cells are not restricted to the somites or limb buds during early embryo development and can move around the embryo (De Angelis et al, 1999; Asakura et al, 2002). Satellite cells proliferate in response to stimuli such as exercise and injury, and descendents undergo several rounds of cell division before differentiating into new multinucleate muscle fibres (reviewed in Grounds and Yablonka-Reuveni, 1993; Bischoff, 1994; Buckingham, 2001).
PAX7 expression is implicated in the specification and maintenance of satellite cells, and here we have shown that PAX7 expression is almost completely restricted to RMS tumours with no known translocation. We therefore propose that these tumours may be derived from the myogenic satellite cell lineage. This is consistent with the analysis of MET expression by conventional RT–PCR, which shows expression of MET mRNA in all 19 tumours with no known translocation: quiescent satellite cells normally express MET, the receptor for hepatocyte growth factor, prior to entering S-phase (Cornelison and Wold, 1997). MET (c-met) is generally believed to be a downstream target of PAX3, and it is unexpected to find that MET is only expressed in four out of nine cases of ARMS. However, Epstein et al (1996) have previously showed that PAX3 and PAX3-FKHR expression only modestly increases expression of c-met, and that PAX3-FKHR is not sufficient in all cases to activate MET.
A recent mouse model for RMS has been described by Sharp et al (2002) who report that HGF/SF Ink4A/Arf−/− mice develop multifocal sarcomatous malignancies arising from trunk and limb skeletal muscles. In histopathological analysis, the tumours resembled human ERMS, expressing low Pax3 levels, and elevated Pax7 levels, which generally mirrored c-Met expression. They describe the earlier appearance of hyperplastic satellite cells in the skeletal muscle of these mice, and suggest that these myogenic precursors were the source of the RMS tumours. The authors propose that aberrant activation of the Pax7/c-Met pathways may induce formation of ectopic skeletal muscle by encouraging satellite-cell specification at the expense of alternate developmental stem cell programmes.
These data from a novel mouse model of ERMS support our proposal that in cases of human ERMS, elevated PAX7 expression and consistent MET expression indicate an origin in myogenic satellite cells for this subtype of RMS. The inherent on/off proliferative capacity of these cells may increase their susceptibility to transformation, and a distinct cell origin for this subtype of RMS could explain its different behaviour and prognosis. Molecular profiling of myogenic genes in satellite cells and RMS with and without PAX-FKHR fusion genes should further define the relation between these cell populations and provide insights into the histogenesis and variable clinical behaviour of the different subtypes of RMS.
Acknowledgments
We thank ER Lawlor (UCSF) and JD Hyer (UCSF) for their critical reading of the manuscript. NT was supported by the Association for International Cancer Research and the Overseas Research Students Award scheme (Universities UK). This work was funded in part by the Cancer Research Campaign and the Royal Marsden Hospital Children's Cancer Unit Fund.
References
- Anderson J, Gordon A, Pritchard-Jones K, Shipley J (1999) Genes, chromosomes and rhabdomyosarcoma. Genes Chromosomes Cancer 26: 275–285 [PubMed] [Google Scholar]
- Anderson J, Gordon T, McManus A, Mapp T, Gould S, Kelsey A, McDowell H, Pinkerton R, Shipley J, Pritchard-Jones K (2001) Detection of the PAX3-FKHR fusion gene in paediatric rhabdomyosarcoma: a reproducible predictor of outcome? Br J Cancer 85: 831–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA (2002) Myogenic specification of side population cells in skeletal muscle. J Cell Biol 159: 123–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr FG (1999) The role of chimeric paired box transcription factors in the pathogenesis of pediatric rhabdomyosarcoma. Cancer Res 59: 1711s–1715s [PubMed] [Google Scholar]
- Barr FG (2001) Gene fusions involving PAX and FOX family members in alveolar rhabdomyosarcoma. Oncogene 20: 5736–5746 [DOI] [PubMed] [Google Scholar]
- Barr FG, Fitzgerald JC, Ginsberg JP, Vanella ML, Davis RJ, Bennicelli JL (1999) Predominant expression of alternative PAX3 and PAX7 forms in myogenic and neural tumor cell lines. Cancer Res 59: 5443–5448 [PubMed] [Google Scholar]
- Barr FG, Nauta LE, Davis RJ, Schafer BW, Nycum LM, Biegel JA (1996) In vivo amplification of the PAX3-FKHR and PAX7-FKHR fusion genes in alveolar rhabdomyosarcoma. Hum Mol Genet 5: 15–21 [DOI] [PubMed] [Google Scholar]
- Bennicelli JL, Edwards RH, Barr FG (1996) Mechanism for transcriptional gain of function resulting from chromosomal translocation in alveolar rhabdomyosarcoma. Proc Natl Acad Sci USA 93: 5455–5459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernasconi M, Remppis A, Fredericks WJ, Rauscher FJ, Schafer BW (1996) Induction of apoptosis in rhabdomyosarcoma cell through down-regulation of PAX proteins. Proc Natl Acad Sci USA 93: 13164–13169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R (1994) The satellite cell and muscle regeneration. In Myogenesis, Engel AG, Franszini-Armstrong C (eds) pp. 97–118. New York: McGraw-Hill [Google Scholar]
- Bober E, Franz T, Arnold HH, Gruss P, Tremblay P (1994) Pax-3 is required for the development of limb muscles: a possible role for the migration of dermomyotomal muscle progenitor cells. Development 120: 603–612 [DOI] [PubMed] [Google Scholar]
- Borycki A-G, Li J, Emerson Jr CP, Epstein JA (1999) Pax3 functions in cell survival and in pax7 regulation. Development 126: 1665–1674 [DOI] [PubMed] [Google Scholar]
- Buckingham M (2001) Skeletal muscle formation in vertebrates. Curr Opin Genet Dev 11: 440–448 [DOI] [PubMed] [Google Scholar]
- Cornelison DD, Wold BJ (1997) Single cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol 191: 270–283 [DOI] [PubMed] [Google Scholar]
- De Angelis L, Berghella L, Coletta M, Lattanzi L, Zanchi M, Cusella-De Angelis MG, Ponzetto C, Cossu G (1999) Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. J Cell Biol 147: 869–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JA, Lam P, Jepeal L, Maas RL, Shapiro DN (1995) Pax3 inhibits myogenic differentiation of cultured myoblast cells. J Biol Chem 270: 11719–11722 [DOI] [PubMed] [Google Scholar]
- Epstein JA, Shapiro DN, Cheng J, Lam PYP, Maas RL (1996) Pax3 modulates expression of the c-Met receptor during limb muscle development. Proc Natl Acad Sci USA 93: 4213–4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JC, Scherr AM, Barr FG (2000) Structural analysis of PAX7 rearrangements in alveolar rhabdomyosarcoma. Cancer Genet Cytogenet 117: 37–40 [DOI] [PubMed] [Google Scholar]
- Fredericks WJ, Galili N, Mukhopadhyay S, Rovera G, Bennicelli J, Barr FG, Rauscher III FJ (1995) The PAX3-FKHR fusion protein created by the t(2;13) translocation in alveolar rhabdomyosarcomas is a more potent transcriptional activator than PAX3. Mol Cell Biol 15: 1522–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg JP, Davis RJ, Bennicelli JL, Nauta LE, Barr FG (1998) Up-regulation of MET but not neural cell adhesion molecule expression by the PAX3-FKHR fusion protein in alveolar rhabdomyosarcoma. Cancer Res 58: 3542–3546 [PubMed] [Google Scholar]
- Gordon T, McManus A, Anderson J, Min T, Swansbury J, Pritchard-Jones K, Shipley J (2001) Cytogenetic abnormalities in 42 rhabdomyosarcoma: a United Kingdom Cancer Cytogenetics Group Study. Med Pediatr Oncol 36: 259–267 [DOI] [PubMed] [Google Scholar]
- Goulding M, Lumsden A, Paquette AJ (1994) Regulation of Pax-3 expression in the dermomyotome and its role in muscle development. Development 120: 957–971 [DOI] [PubMed] [Google Scholar]
- Grounds MD, Yablonka-Reuveni Z (1993) Molecular and cell biology of skeletal muscle regeneration. Mol Cell Biol Hum Dis Ser 3: 210–256 [DOI] [PubMed] [Google Scholar]
- Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC (1999) Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 401: 390–394 [DOI] [PubMed] [Google Scholar]
- Hollowood K, Fletcher CD (1994) Rhabdomyosarcoma in adults. Semin Diagn Pathol 11: 47–57 [PubMed] [Google Scholar]
- Jackson KA, Mi T, Goodell MA (1999) Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci USA 96: 14482–14486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri A (1998) The role of Pax3 and Pax7 in development and cancer. Crit Rev Oncog 9: 141–149 [DOI] [PubMed] [Google Scholar]
- Maulbecker CC, Gruss P (1993) The oncogenic potential of Pax genes. EMBO J 12: 2361–2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlino G, Helman LJ (1999) Rhabdomyosarcoma–working out the pathways. Oncogene 18: 5340–5348 [DOI] [PubMed] [Google Scholar]
- Schiedler S, Fredericks WJ, Rauscher III FJ, Barr FG, Vogt PK (1996) The hybrid PAX3-FKHR fusion protein of alveolar rhabdomyo-sarcoma transforms fibroblasts in culture. Proc Natl Acad Sci USA 93: 9805–9809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Rudnicki MA (2000) A new look at the origin, function, and ‘stem-cell’ status of muscle satellite cells. Dev Biol 218: 115–124 [DOI] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA (2000) Pax7 is required for the specification of myogenic satellite cells. Cell 102: 777–786 [DOI] [PubMed] [Google Scholar]
- Sharp R, Recio JA, Jhappan C, Otsuka T, Liu S, Yu Y, Liu W, Anver M, Navid F, Helman LJ, DePinho RA, Merlino G (2002) Synergism between INK4a/ARF inactivation and aberrant HGF/SF signalling in rhabdomyosarcomagenesis. Nat Med 8: 1276–1280 [DOI] [PubMed] [Google Scholar]
- Weber-Hall S, Anderson J, McManus A, Abe S, Nojima T, Pinkerton R, Pritchard-Jones K, Shipley J (1996) Gains, losses, and amplification of genomic material in rhabdomyosarcoma analyzed by comparative genomic hybridization. Cancer Res 56: 3220–3224 [PubMed] [Google Scholar]