Abstract
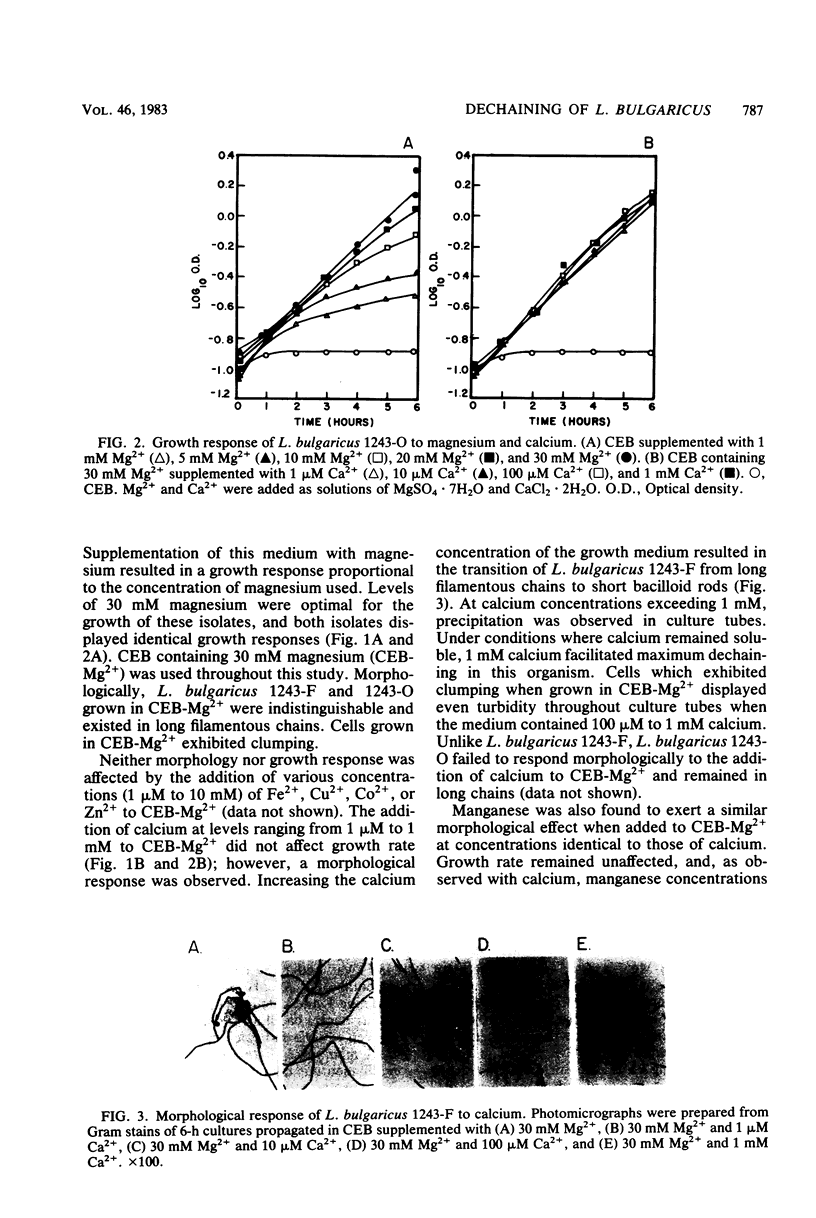
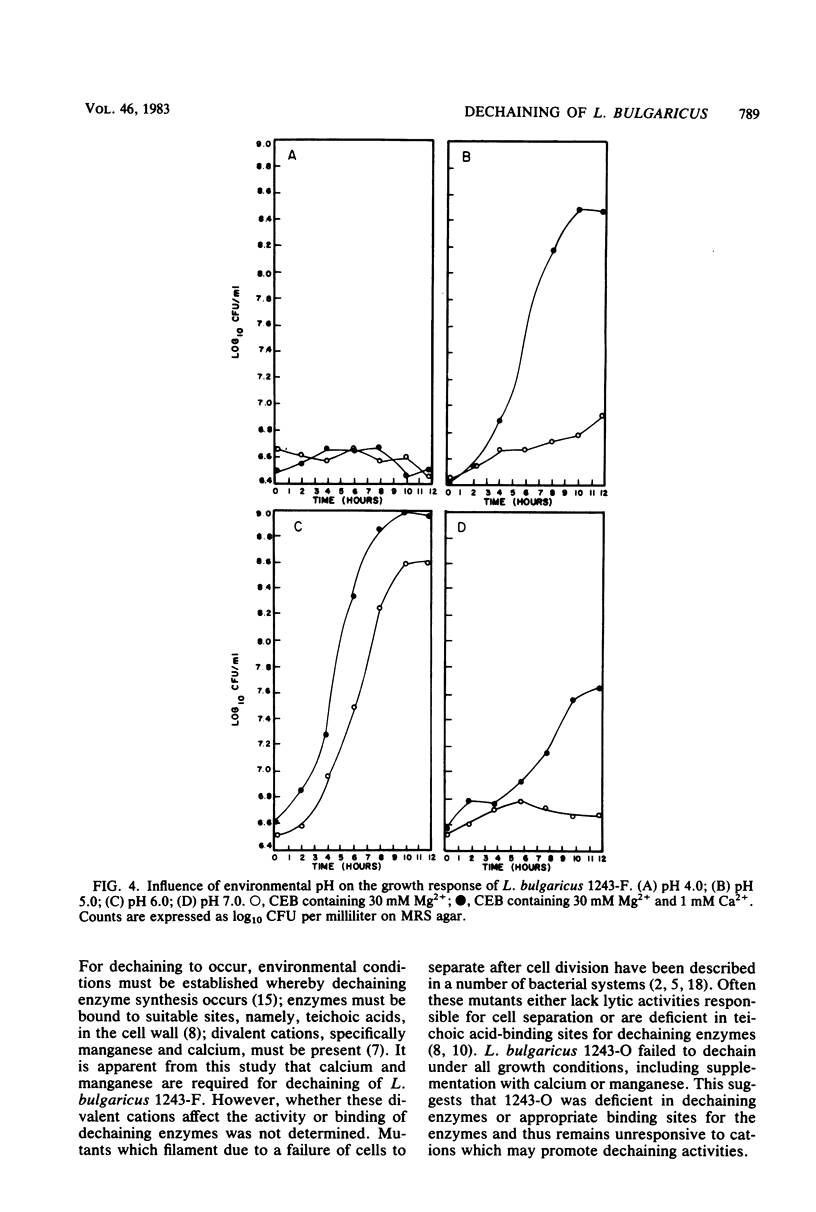
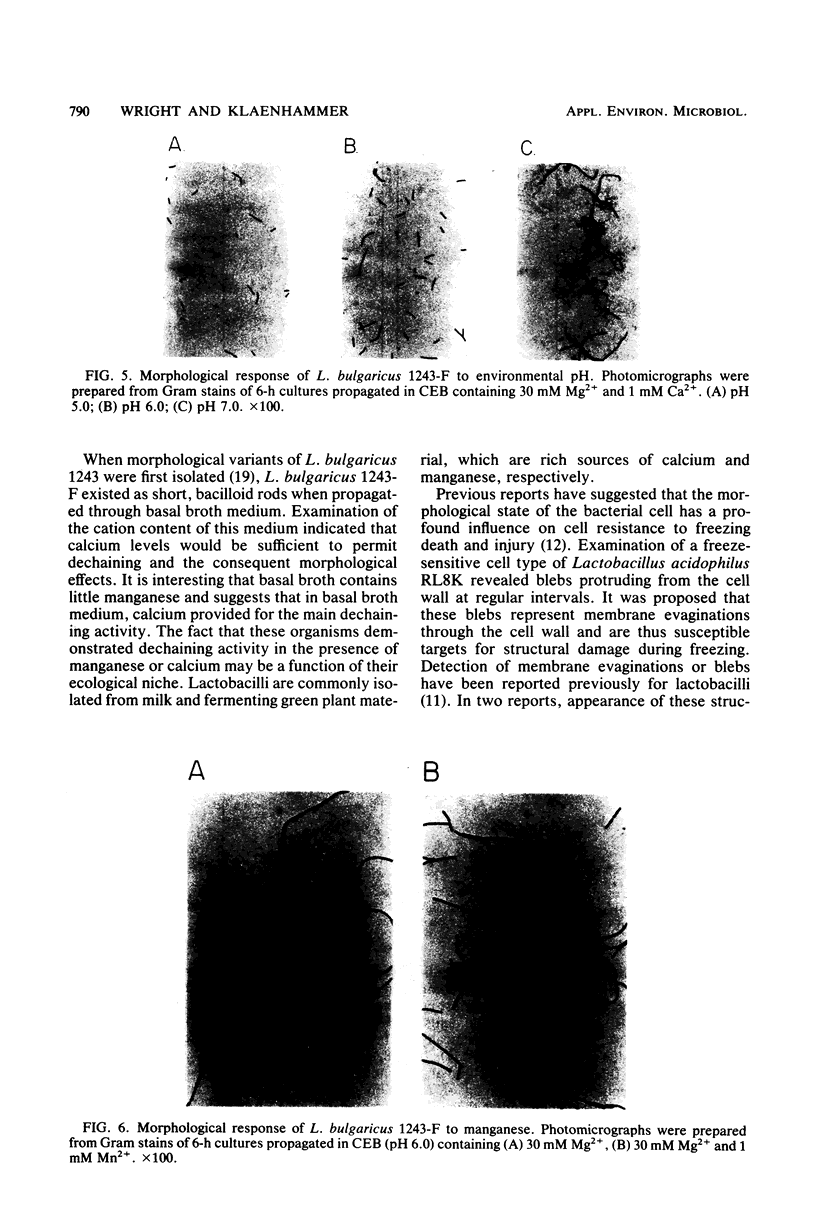
The events responsible for the transition of Lactobacillus bulgaricus 1243-F from long filamentous chains to short bacilloid rods were examined in a cation-depleted liquid medium. In the presence of magnesium only, cells grew as long chains of unseparated cells. The addition of 100 μM to 1 mM calcium or manganese to this medium resulted in the dechaining of these cells to short bacilloid rods. Fe2+, Zn2+, Co2+, and Cu2+ failed to induce dechaining. Induction of calcium and manganese dechaining functioned under controlled pH maintained at 5.0 and 6.0 but not at pH 7.0. This was consistent with a previous report showing failure in synthesis of dechaining enzymes by L. bulgaricus under pH conditions approaching alkalinity (S. K. Rhee and M. Y. Pack, J. Bacteriol. 144:865-868, 1980). We conclude that under pH conditions which permit synthesis of dechaining enzymes, calcium and manganese are necessary for dechaining activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber F. W., Frazier W. C. Dissociants of Lactobacilli. J Bacteriol. 1945 Dec;50(6):637–649. [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. N., Wong W., Young F. E., Gilpin R. W. Isolation and characterization of a mutant of Staphylococcus aureus deficient in autolytic activity. J Bacteriol. 1976 Mar;125(3):961–967. doi: 10.1128/jb.125.3.961-967.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyette J., Shockman G. D. Some properties of the autolytic N-acetylmuramidase of Lactobacillus acidophilus. J Bacteriol. 1973 Apr;114(1):34–41. doi: 10.1128/jb.114.1.34-41.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P. Autolysin(s) of Bacillus subtilis as dechaining enzyme. J Bacteriol. 1970 Aug;103(2):494–499. doi: 10.1128/jb.103.2.494-499.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Rogers H. J. Characterization of Bacillus licheniformis 6346 mutants which have altered lytic enzyme activities. J Bacteriol. 1974 May;118(2):358–368. doi: 10.1128/jb.118.2.358-368.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRULA E. A., GRULA M. M. Cell division in a species of Erwinia III. Reversal of inhibition of cell division caused by D-amino acids, penicillin, and ultraviolet light. J Bacteriol. 1962 May;83:981–988. doi: 10.1128/jb.83.5.981-988.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbold D. R., Glaser L. Bacillus subtilis N-acetylmuramic acid L-alanine amidase. J Biol Chem. 1975 Mar 10;250(5):1676–1682. [PubMed] [Google Scholar]
- Herbold D. R., Glaser L. Interaction of N-acetylmuramic acid L-alanine amidase with cell wall polymers. J Biol Chem. 1975 Sep 25;250(18):7231–7238. [PubMed] [Google Scholar]
- Higgins M. L., Coyette J., Shockman G. D. Sites of cellular autolysis in Lactobacillus acidophilus. J Bacteriol. 1973 Dec;116(3):1375–1382. doi: 10.1128/jb.116.3.1375-1382.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst A., Stubbs J. M. Electron microscopic study of membranes and walls of bacteria and changes occurring during growth initiation. J Bacteriol. 1969 Mar;97(3):1466–1479. doi: 10.1128/jb.97.3.1466-1479.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Specific recognition of choline residues in the cell wall teichoic acid by the N-acetylmuramyl-L-alanine amidase of Pneumococcus. J Biol Chem. 1975 Aug 10;250(15):6072–6076. [PubMed] [Google Scholar]
- Klaenhammer T. R., Kleeman E. G. Growth Characteristics, Bile Sensitivity, and Freeze Damage in Colonial Variants of Lactobacillus acidophilus. Appl Environ Microbiol. 1981 Jun;41(6):1461–1467. doi: 10.1128/aem.41.6.1461-1467.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M., Suda S., Hotta S., Hamada K., Suganuma A. Necessity of calcium ion for cell division in Lactobacillus bifidus. J Bacteriol. 1970 Nov;104(2):1010–1013. doi: 10.1128/jb.104.2.1010-1013.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. K., Pack M. Y. Effect of environmental pH on chain length of lactobacillus bulgaricus. J Bacteriol. 1980 Dec;144(3):865–868. doi: 10.1128/jb.144.3.865-868.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozzi T., Maret R., Poulin J. M. Study of plating efficiency of bacteriophages of thermophilic lactic acid bacteria on different media. Appl Environ Microbiol. 1976 Jul;32(1):131–137. doi: 10.1128/aem.32.1.131-137.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf-Watz H., Normark S. Evidence for a role of N-acetylmuramyl-L-alanine amidase in septum separation in Escherichia coli. J Bacteriol. 1976 Nov;128(2):580–586. doi: 10.1128/jb.128.2.580-586.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]