Abstract
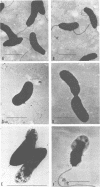
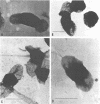
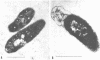
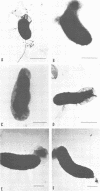
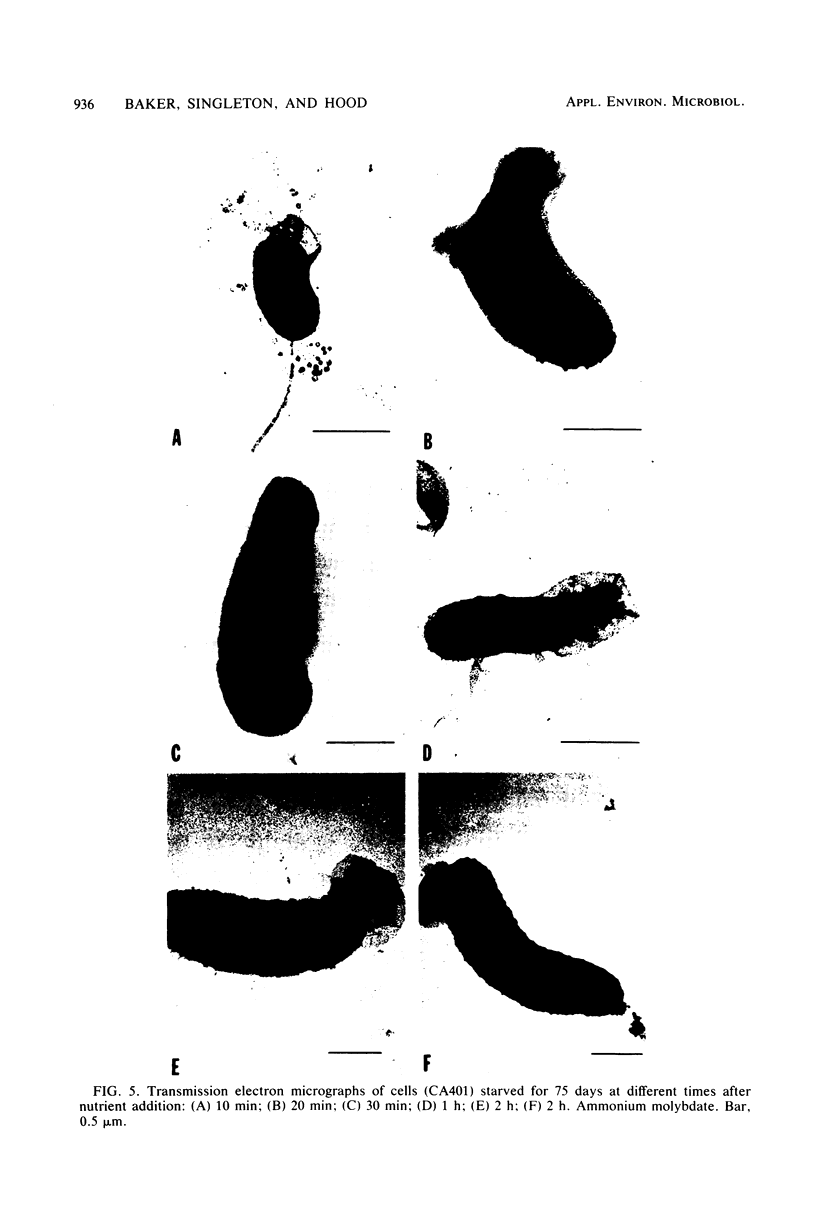
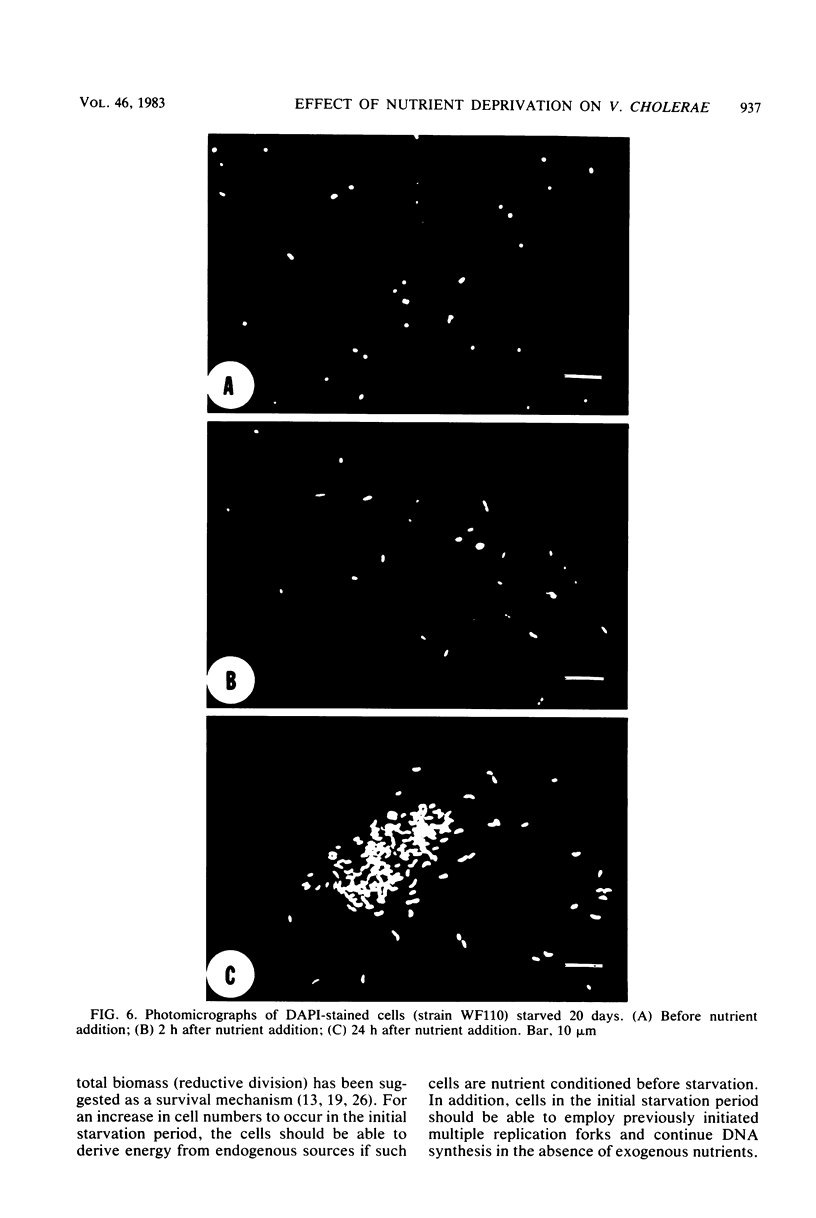
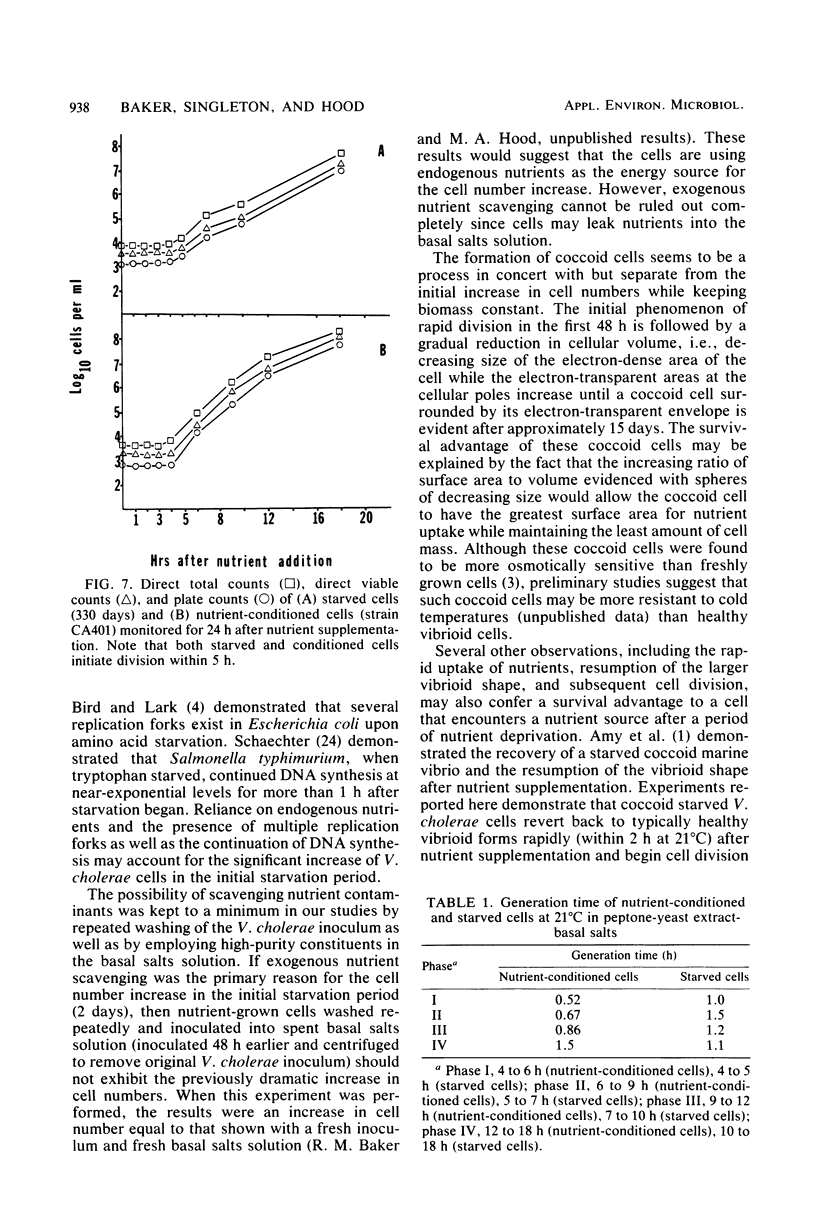
Environmental and clinical strains of Vibrio cholerae were exposed to nutrient-free artificial seawater and filtered natural seawater microcosms for selected time intervals and examined for changes in cell morphology and number. Cells observed by transmission electron and epifluorescence microscopy were found to undergo gross alterations in cell morphology with time of exposure. The vibroid cells decreased in volume by 85% and developed into small coccoid forms surrounded by remnant cell walls. The initial number of cells inoculated into nutrient-free microcosms (culturable count and direct viable count) increased 2.5 log10 within 3 days, and even after 75 days the number of viable cells was still 1 to 2 log10 higher than the initial inoculum size. Nutrient-depleted coccoid-shaped cells were restored to normal size and assumed a bacillary shape within 3 h and began to divide within 5 h after nutrient supplementation. The increase in cell number and decrease in cell volume under nutrient-depleted conditions, as well as the rapid growth response after nutrient supplementation, may describe some of the survival mechanisms of V. cholerae in the aquatic environment.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amy P. S., Pauling C., Morita R. Y. Recovery from nutrient starvation by a marine Vibrio sp. Appl Environ Microbiol. 1983 May;45(5):1685–1690. doi: 10.1128/aem.45.5.1685-1690.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D. A., Park R. W. Changes in morphology and cell wall structure that occur during growth of Vibrio sp. NCTC4716 in batch culture. J Gen Microbiol. 1975 Jan;86(1):12–28. doi: 10.1099/00221287-86-1-12. [DOI] [PubMed] [Google Scholar]
- Bird R., Lark K. G. Initiation and termination of DNA replication after amino acid starvation of E. coli 15T-. Cold Spring Harb Symp Quant Biol. 1968;33:799–808. doi: 10.1101/sqb.1968.033.01.092. [DOI] [PubMed] [Google Scholar]
- CARLUCCI A. F., PRAMER D. Factors affecting the survival of bacteria in sea water. Appl Microbiol. 1959 Nov;7:388–392. doi: 10.1128/am.7.6.388-392.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell R. R., Seidler R. J., Kaper J., Joseph S. W., Garges S., Lockman H., Maneval D., Bradford H., Roberts N., Remmers E. Occurrence of Vibrio cholerae serotype O1 in Maryland and Louisiana estuaries. Appl Environ Microbiol. 1981 Feb;41(2):555–558. doi: 10.1128/aem.41.2.555-558.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J., Lockman H., Colwell R. R., Joseph S. W. Ecology, serology, and enterotoxin production of Vibrio cholerae in Chesapeake Bay. Appl Environ Microbiol. 1979 Jan;37(1):91–103. doi: 10.1128/aem.37.1.91-103.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelleberg S., Humphrey B. A., Marshall K. C. Effect of interfaces on small, starved marine bacteria. Appl Environ Microbiol. 1982 May;43(5):1166–1172. doi: 10.1128/aem.43.5.1166-1172.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure K., Simidu U., Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979 Mar;25(3):415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- Macdonell M. T., Hood M. A. Isolation and characterization of ultramicrobacteria from a gulf coast estuary. Appl Environ Microbiol. 1982 Mar;43(3):566–571. doi: 10.1128/aem.43.3.566-571.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitsky J. A., Morita R. Y. Morphological characterization of small cells resulting from nutrient starvation of a psychrophilic marine vibrio. Appl Environ Microbiol. 1976 Oct;32(4):617–622. doi: 10.1128/aem.32.4.617-622.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitsky J. A., Morita R. Y. Survival of a psychrophilic marine Vibrio under long-term nutrient starvation. Appl Environ Microbiol. 1977 Mar;33(3):635–641. doi: 10.1128/aem.33.3.635-641.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAECHTER M. Patterns of cellular control during unbalanced growth. Cold Spring Harb Symp Quant Biol. 1961;26:53–62. doi: 10.1101/sqb.1961.026.01.011. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Torrella F., Morita R. Y. Microcultural study of bacterial size changes and microcolony and ultramicrocolony formation by heterotrophic bacteria in seawater. Appl Environ Microbiol. 1981 Feb;41(2):518–527. doi: 10.1128/aem.41.2.518-527.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]