Abstract
Yersinia spp. use a type 3 secretion system (T3SS) to directly inject six proteins into macrophages, and any impairment of this process results in a profound reduction in virulence. We previously showed that the exoribonuclease polynucleotide phosphorylase (PNPase) was required for optimal T3SS functioning in Yersinia pseudotuberculosis and Yersinia pestis. Here we report that Y. pseudotuberculosis cells with reduced RNase E activity are likewise impaired in T3SS functioning and that phenotypically they resemble Δpnp cells. RNase E does not affect expression levels of the T3SS substrates but instead, like PNPase, regulates a terminal event in the secretion pathway. This similarity, together with the fact that RNase E and PNPase can be readily copurified from Y. pseudotuberculosis cell extracts, suggests that these two RNases regulate T3SS activity through a common mechanism. This is the first report that RNase E activity impacts the T3SS as well as playing a more general role in infectivity.
Several species of gram-negative bacteria possess a membrane-bound organelle that exports proteins, referred to as “effectors,” directly into eukaryotic cells (5). Although they were first described in bacterial pathogens, it is now recognized that these protein delivery structures, designated type 3 secretion systems (T3SSs), represent a general communication conduit between bacteria and their hosts (4). T3SS mutants can be divided into two broad classes having defects in either the protein delivery pathway or effector activity within the host cell. We have recently described reduced T3SS function in mutant strains of Yersinia pseudotuberculosis and Yersinia pestis having deletions in the gene encoding the RNase polynucleotide phosphorylase (PNPase) (17). Even though the Δpnp strains of these two animal pathogens had only a partial delivery defect, this reduction in T3SS activity resulted in a significant diminution in their infectivity in both cell culture- and animal-based assays (17, 18). Steady-state expression and inductive expression of T3SS transcripts and proteins were at similar levels in the Δpnp strains and their isogenic wild-type strains, and instead the delivery defect of the former strains appeared to occur at a terminal step in the secretion pathway. Unexpectedly, a catalytically inactive PNPase restored normal T3SS activity to these Δpnp strains; this was especially surprising given the fact that an active PNPase is absolutely required to restore the cold-growth defect of the Yersinia Δpnp strains (17).
Our results suggested that PNPase regulates T3SS activity indirectly and motivated us to examine other factors that are functionally associated with PNPase. One such factor, the endoribonuclease RNase E, plays a role in both initiating transcript degradation and processing tRNAs and rRNAs (8, 9, 10, 15). In Escherichia coli, the amino-terminal region of RNase E is associated with its RNase activity whereas the carboxyl-terminal region serves as a scaffolding platform for PNPase, the helicase RhlB, and enolase, a glycolytic enzyme; this multifactor complex is designated the “degradosome” (3, 11, 16). Here we examine the role of RNase E in the functioning of the T3SS of Y. pseudotuberculosis.
By transcript profiling, we noted that the RNase E-encoding transcript was at elevated levels in the Y. pseudotuberculosis Δpnp strain compared to the wild-type strain (unpublished observations). We therefore investigated whether there is a functional connection between the T3SS, PNPase, and RNase E in Y. pseudotuberculosis. In E. coli RNase E is essential for viability and regulates it own synthesis by degrading its transcript (7, 14). Recently we showed that this autoregulatory activity could be inhibited by expressing a carboxyl-terminally truncated RNase E variant that conferred a dominant-negative phenotype (2). Since the RNase E amino-terminal regions of E. coli and Y. pseudotuberculosis are highly conserved (94% identity over their first 500 amino acids), we took a similar approach for Y. pseudotuberculosis.
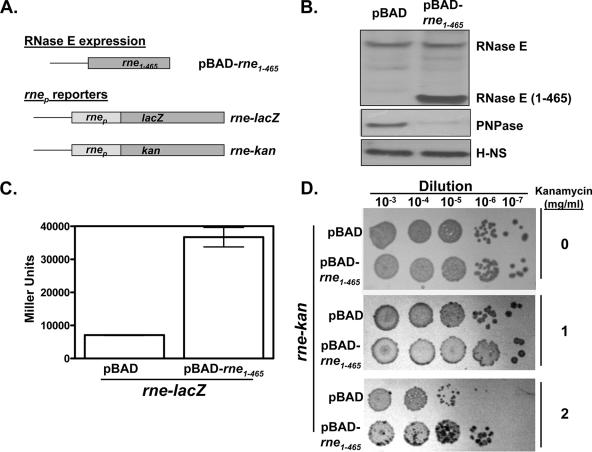
We constructed an expression plasmid encoding a carboxyl-terminally truncated variant of RNase E of Y. pseudotuberculosis under the control of an inducible promoter (pBAD-rne1-465) (Fig. 1A). Y. pseudotuberculosis transformed with this plasmid stably expressed the truncated RNase E, and as is the case for E. coli (2), expression of the truncated RNase E did not affect the levels of endogenous full-length RNase E (Fig. 1B). We also constructed reporter plasmids that give a readout on cellular RNase E activity. These latter plasmids consist of the 5′ untranslated region of rne, which, in E. coli, is targeted by the autoregulatory activity of RNase E (7), controlling the expression of either lacZ or kan (rne-lacZ and rne-kan, respectively [Fig. 1A]). Y. pseudotuberculosis strains cotransformed with rne-lacZ and either a control plasmid (pBAD18) or pBAD-rne1-465 were grown to mid-log phase in the presence of arabinose and subsequently assayed for β-galactosidase activity. Strikingly, β-galactosidase levels of the Y. pseudotuberculosis strain expressing pBAD-rne1-465 were ∼5-fold higher (indicative of reduced RNase E activity) than those of the strain containing the control plasmid (Fig. 1C). No such increase was observed in the absence of arabinose (data not shown), indicating that expression of the truncated RNase E protein is necessary for its inhibitory function. Similarly, Y. pseudotuberculosis strains cotransformed with rne-kan and either the control or pBAD-rne1-465 plasmid were plated on semisolid media containing arabinose and various concentrations of kanamycin. The growth rates of these two strains were comparable at relatively lower kanamycin concentrations (≤1 mg/ml) whereas at higher kanamycin concentrations (2 mg/ml) the pBAD-rne1-465-transformed strain possessed a clear growth advantage over the strain transformed with the control plasmid (Fig. 1D). Taken together, these results show that the Yersinia homolog of the E. coli dominant-negative inhibitor has a similar inhibitory effect on Yersinia RNase E function.
FIG. 1.
Modulating RNase E activity in Y. pseudotuberculosis. (A) The RNase E expression plasmid used in this study contains a region of the Y. pseudotuberculosis RNase E gene, rne1-465, that had previously been shown to exert a dominant-negative phenotype on the autoregulatory activity of RNase E in E. coli (2). pBAD-rne1-465 was created by first using the PCR and Y. pseudotuberculosis genomic DNA as a template to generate a fragment containing the first 465 codons of RNase E that was subsequently cloned into pBAD24. Plasmids that report on the autoregulatory activity of RNase E (“rnep reporters”) contain 510 nucleotides of the upstream promoter region and the first 307 nucleotides of the rne gene of Y. pseudotuberculosis fused in frame with either lacZ or the kanamycin resistance gene from Tn903 in a pACYC-based vector. (B) Western blots were prepared from whole-cell lysates of the indicated Y. pseudotuberculosis transformants and probed with antisera recognizing RNase E, PNPase E, and H-NS (the last serving as a loading control). (C) Y. pseudotuberculosis strains cotransformed with the rne-lacZ reporter and either the control or the RNase E1-465 expression plasmid were propagated in arabinose-containing medium prior to being collected and assayed for β-galactosidase activity (12). (D) Y. pseudotuberculosis strains cotransformed with the rne-kan reporter and either the control or the RNase E1-465 expression plasmid were propagated as for panel C and then plated on medium containing arabinose and the indicated concentration of kanamycin. For unknown reasons Y. pseudotuberculosis forms darker colonies in the presence of kanamycin.
The reporters described above were also used to compare the RNase E activities of the wild-type and Δpnp Y. pseudotuberculosis strains. The β-galactosidase activity of liquid-grown Δpnp cells transformed with rne-lacZ was ∼2.5-fold higher than that of wild-type cells; wild-type-like rne-lacZ activity was restored to Δpnp cells by expressing PNPase in trans (Fig. 2A). Similarly, the Δpnp strain transformed with rne-kan grew at higher kanamycin levels than did the likewise-transformed wild-type strain (data not shown). These findings are consistent with the above-mentioned observation of elevated rne transcripts in the Δpnp strain compared to the wild-type strain. Conversely, we also found reduced PNPase protein levels in cells expressing RNase E1-465 (Fig. 1B), further suggesting the functional linkage between these two RNases. These findings motivated us to test whether RNase E and PNPase physically interact with one another in Y. pseudotuberculosis similarly to what has been described for E. coli. We therefore expressed and isolated hemagglutinin (HA)-tagged PNPase from Y. pseudotuberculosis lysates and probed for RNase E in the HA-PNPase-enriched fraction. RNase E was readily detected in HA-PNPase immunoprecipitates, as with E. coli by a similar approach (Fig. 2B; data for E. coli not shown). Collectively, these data indicate that there is both a functional and a physical relationship between RNase E and PNPase in Y. pseudotuberculosis.
FIG. 2.
Function and physical interaction of RNase E and PNPase in Y. pseudotuberculosis. (A) Wild-type (wt) Y. pseudotuberculosis and its Δpnp derivative were cotransformed with the rne-lacZ reporter and the indicated expression plasmids and assayed for β-galactosidase activity as described for Fig. 1. (B) The Y. pseudotuberculosis Δpnp strain transformed with an inducibly expressed HA-tagged PNPase-encoding plasmid was propagated either in the presence or in the absence of arabinose. Immunoprecipitations were performed starting with 50-ml cultures grown to an optical density at 600 nm of 0.6 and induced for HA-PNPase expression for 1 h prior to harvesting. Cells were lysed in phosphate-buffered saline supplemented with protease inhibitor cocktail (Sigma-Aldrich) and 1 mM dithiothreitol by two passages through a French press. Cleared lysates were diluted 10-fold with HB (25 mM MOPS [morpholinepropanesulfonic acid], pH 7.2, 60 mM glycerol 2-phosphate disodium salt hydrate, 15 mM 4-nitrophenyl phosphate disodium salt hexahydrate, 15 mM MgCl2, 15 mM EGTA, 0.1 mM sodium orthovanadate, 1% Triton), and anti-HA antibody (HA.11; Covance Research Products) was added. Following 1.5 h of incubation at 4°C, HB-washed ProG magnetic beads (New England Biolabs) were added and the samples were further incubated for 2 h. Beads were washed extensively with phosphate-buffered saline-dithiothreitol (three times for 15 min each) and were resuspended in sodium dodecyl sulfate loading buffer and fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The resulting Western blot was separately probed with PNPase-, RNase E-, and H-NS-specific antisera.
Compared to its isogenic wild-type strain, the Y. pseudotuberculosis Δpnp strain has a reduced level of infectivity in both cell culture and animal infection assays (17, 18). In light of our findings of the interrelationship between PNPase and RNase E, we examined whether reduced RNase E activity would similarly be accompanied by a reduction in infectivity. Infectivity was assessed using an assay in which Y. pseudotuberculosis strains are added to cultured macrophages and at various times thereafter viable macrophage-associated bacteria are enumerated by plating (1). In the representative experiment shown in Fig. 3, there was a 69-fold increase in the number of Y. pseudotuberculosis bacteria transformed with the control plasmid following a 6-h infection period. In contrast, a Y. pseudotuberculosis T3SS mutant strain, the ΔyopB strain, in which the Yop effectors are expressed and secreted but not transferred into the macrophage (6), increased only 1.8-fold during the 6-h infection period. The Y. pseudotuberculosis strain transformed with pBAD-rne1-465 increased 25-fold during the 6-h infection period. This intermediate level of infectivity, relative to the wild-type and ΔyopB strains, is similar to what was previously observed for the Y. pseudotuberculosis Δpnp strain (17). Expression of the truncated RNase E did not affect the bacterial growth rate in the absence of macrophages (Fig. 3, inset). This latter finding suggests that, as for the Y. pseudotuberculosis ΔyopB strain, the reduction in infectivity of the pBAD-rne1-465-transformed Y. pseudotuberculosis strain is due to a defect in the “survival” component of the “infectivity = survival + growth” equation.
FIG. 3.
RNase E activity and Y. pseudotuberculosis infectivity. Wild-type (wt) Y. pseudotuberculosis and its ΔyopB derivative, transformed with the indicated plasmids, were propagated in tissue culture medium supplemented with arabinose prior to being added to wells containing macrophage-like RAW 267 cells at a multiplicity of infection of ∼1. Following a 30-min attachment period, excess bacteria were removed, and either immediately (“0 h”) or 6 h later the number of viable cell-associated bacteria per well was determined by plating as previously described (1, 17). The average number of CFU recovered from three independent wells per condition is plotted, and the differences (n-fold) between the 0- and 6-h recoveries are shown above the graph. (Inset) Growth rates of the same strains in tissue culture medium in the absence of macrophages (circles, wt strain with pBAD; squares, ΔyopB strain with pBAD; diamonds, wt strain with pBAD-rne1-465). OD, optical density.
Since there is a well-established link between infectivity and the T3SS in the yersiniae, T3SS activity was assayed in the pBAD-rne1-465-transformed Y. pseudotuberculosis strain. This strain was propagated in conditions that maximize inductive T3SS expression and secretion. Specifically, strains were grown to mid-log phase in “preinductive” conditions (i.e., 37°C in medium containing Ca2+ with or without arabinose) and Yop expression and secretion were triggered by adding a Ca2+ chelator to the culture medium. Prior to triggering of Yop expression and secretion, there were similar levels of YopE (and green fluorescent protein [GFP] [see below]) in the whole-cell fraction irrespective of the presence of arabinose (Fig. 4A, compare lanes 1 and 4). Similarly, following the chelation of Ca2+, comparable levels of YopE (and GFP) were observed in the whole-cell fractions prepared from this strain whether propagated in arabinose or not (compare lanes 2 and 3 with lanes 5 and 6 in Fig. 4A). However, the presence of arabinose had a negative effect on the levels of YopE detected in the supernatant fractions of the pBAD-rne1-465-transformed strain. These results indicate that RNase E activity is specifically important for YopE secretion.
FIG. 4.
RNase E activity and T3SS function. (A) Wild-type Y. pseudotuberculosis transformed with the RNase E1-465 expression plasmid was propagated at 37°C in medium containing 2.5 mM Ca2+ either in the absence or in the presence of arabinose. T3SS expression and secretion were induced by adding a twofold molar excess of EGTA. At the indicated time points, samples were removed, separated into whole-cell and supernatant fractions, and analyzed for YopE and GFP levels by Western blotting. (This strain contains a yopE::gfp transcriptional fusion at the yopE locus [1].) (B) Wild-type Y. pseudotuberculosis transformed with either the control plasmid or RNase E1-465 expression plasmid vector was propagated as described for panel A, and at the indicated time points following the addition of EGTA, cells were removed from the culture and analyzed by fluorescence microscopy as previously described (20). In each column is plotted the mean fluorescence intensity (MFI) of every bacterial cell in a single field (4 to 10 fields/2,036 to 2,389 total cells per sampling point). Bars represent the population means, and the slopes calculated from the 60-, 90-, and 120-min mean values are 3.3 and 3.0 MFI/min for the pBAD- and pBAD-rne1-465-transformed Y. pseudotuberculosis, respectively.
The Y. pseudotuberculosis strain used for these studies contains a gfp gene inserted between the yopE stop and transcriptional termination sequences; this alteration has no detectable consequence either for inductive YopE expression or in the relative performance of the strain in cell culture and animal infection models (1). It is important to note that, unlike YopE, GFP is not a substrate for the T3SS and is retained within the cell following its synthesis, thus serving, as shown in Fig. 4A, as a reporter for yopE promoter activity and message levels. Using this strain, we have recently developed a high-throughput microscopy protocol to analyze inductive yopE expression in single cells (20). Using methods of propagation and induction identical to those described for Fig. 4A, we measured GFP levels in single Y. pseudotuberculosis cells from strains transformed with either the control plasmid or pBAD-rne1-465. Individual cells from these strains displayed nearly identical patterns of increase in their fluorescence in response to the chelation of Ca2+ (Fig. 4B and its legend). Specifically, both the absolute fluorescence intensities and the rates of increase in signal calculated from the mean fluorescence intensities were comparable between these two strains, indicating that RNase E affects neither inductive nor steady-state Yop expression levels.
Collectively, the findings presented here support a model in which the reduction in infectivity of a Y. pseudotuberculosis strain with attenuated RNase E function is due to a defect in the T3SS. Based on the similar phenotypes of RNase E1-465-expressing and Δpnp Y. pseudotuberculosis strains in infectivity and Yop secretion assays (17, 18), as well as the functional and physical linkage between RNase E and PNPase (Fig. 1B and 2), we propose that RNase E and PNPase affect T3SS activity through a common pathway. If true, it would be expected that there would be no additive negative effects on T3SS function of combining reduced RNase E and PNPase activities in a single strain. Unfortunately this prediction could not be directly tested due to the fact that the pBAD-rne1-465-transformed Y. pseudotuberculosis Δpnp strain had a somewhat modest growth defect (vis-à-vis the pnp+ strain) when propagated at 26°C which became notably exacerbated at the “preinductive” condition of 37°C.
By what means could these RNases control T3SS activity? One obvious possibility is that RNase E/PNPase could regulate the levels of T3SS-encoding transcripts as has been previously shown for the pap operon of E. coli (13). RNase E is clearly not affecting YopE expression levels (Fig. 4), and by transcript profiling as well as a proteomics-based analysis, T3SS mRNAs and proteins were found to be present at comparable levels in wild-type and Δpnp yersiniae (18). We favor a model in which RNase E/PNPase control the levels of some other transcripts required for optimal T3SS functioning. Viegas et al. have recently shown that, in Salmonella enterica strains that either lack PNPase or express a truncated RNase E, a number of regulatory RNAs are elevated (19). Our findings draw attention to the importance of posttranscriptional processes in infectivity.
Acknowledgments
We thank David Wiley, Padma Sarvepalli, Jason Rosenzweig, Sara Schesser Bartra, and Julia Ross for outstanding technical assistance and Bernt Eric Uhlin and Ken Fields for excellent counsel.
This work was supported by the Department of Microbiology and Immunology, University of Miami, Miller School of Medicine, and Public Health Service grant R01 AI53459 from the National Institutes of Health.
Footnotes
Published ahead of print on 21 March 2008.
REFERENCES
- 1.Bartra, S., P. Cherepanov, Å. Forsberg, and K. Schesser. 2001. The Yersinia YopE and YopH type III effector proteins enhance bacterial proliferation following contact with eukaryotic cells. BMC Microbiol. 122-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briegel, K. J., A. Baker, and C. Jain. 2006. Identification and analysis of Escherichia coli ribonuclease E dominant-negative mutants. Genetics 1727-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carpousis, A. J., G. Van Houwe, C. Ehretsmann, and H. M. Krisch. 1994. Copurification of E. coli RNAase E and PNPase: evidence for a specific association between two enzymes important in RNA processing and degradation. Cell 76889-900. [DOI] [PubMed] [Google Scholar]
- 4.Dale, C., and N. A. Moran. 2006. Molecular interactions between bacterial symbionts and their hosts. Cell 126453-465. [DOI] [PubMed] [Google Scholar]
- 5.Galán, J. E., and H. Wolf-Watz. 2006. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444567-573. [DOI] [PubMed] [Google Scholar]
- 6.Hakansson, S., K. Schesser, C. Persson, E. E. Galyov, R. Rosqvist, F. Homble, and H. Wolf-Watz. 1996. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 155812-5823. [PMC free article] [PubMed] [Google Scholar]
- 7.Jain, C., and J. G. Belasco. 1995. RNase E autoregulates its synthesis by controlling the degradation rate of its own mRNA in Escherichia coli: unusual sensitivity of the rne transcript to RNase E activity. Genes Dev. 984-96. [DOI] [PubMed] [Google Scholar]
- 8.Li, Z., S. Pandit, and M. P. Deutscher. 1999. RNase G (CafA protein) and RNase E are both required for the 5′ maturation of 16S ribosomal RNA. EMBO J. 182878-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li, Z., and M. P. Deutscher. 2002. RNase E plays an essential role in the maturation of Escherichia coli tRNA precursors. RNA 897-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massé, E., F. E. Escorcia, and S. Gottesman. 2003. Coupled degradation of a small regulatory RNA and its mRNA targets in Escherichia coli. Genes Dev. 172374-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miczak, A., V. R. Kaberdin, C. L. Wei, and S. Lin-Chao. 1996. Proteins associated with RNase E in a multicomponent ribonucleolytic complex. Proc. Natl. Acad. Sci. USA 933865-3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller, J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 13.Naureckiene, S., and B. E. Uhlin. 1996. In vitro analysis of mRNA processing by RNase E in the pap operon of Escherichia coli. Mol. Microbiol. 2155-68. [DOI] [PubMed] [Google Scholar]
- 14.Ono, M., and M. Kuwano. 1979. A conditional lethal mutation in an Escherichia coli strain with a longer chemical lifetime of messenger RNA. J. Mol. Biol. 129343-357. [DOI] [PubMed] [Google Scholar]
- 15.Ow, M. C., and S. R. Kushner. 2002. Initiation of tRNA maturation by RNase E is essential for cell viability in E. coli. Genes Dev. 161102-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Py, B., C. F. Higgins, H. M. Krisch, and A. J. Carpousis. 1996. A DEAD-box RNA helicase in the Escherichia coli RNA degradosome. Nature 381169-172. [DOI] [PubMed] [Google Scholar]
- 17.Rosenzweig, J. A., G. Weltman, G. V. Plano, and K. Schesser. 2005. Modulation of yersinia type three secretion system by the S1 domain of polynucleotide phosphorylase. J. Biol. Chem. 280156-163. [DOI] [PubMed] [Google Scholar]
- 18.Rosenzweig, J. A., B. Chromy, A. Echeverry, J. Yang, B. Adkins, G. V. Plano, S. McCutchen-Maloney, and K. Schesser. 2007. Polynucleotide phosphorylase independently controls virulence factor expression levels and export in Yersinia spp. FEMS Microbiol. Lett. 270255-264. [DOI] [PubMed] [Google Scholar]
- 19.Viegas, S. C., V. Pfeiffer, A. Sittka, I. J. Silva, J. Vogel, and C. M. Arraiano. 2007. Characterization of the role of ribonucleases in Salmonella small RNA decay. Nucleic Acids Res. 357651-7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiley, D. J., R. Rosqvist, and K. Schesser. 2007. Induction of the Yersinia type 3 secretion system as an all-or-none phenomenon. J. Mol. Biol. 37327-37. [DOI] [PMC free article] [PubMed] [Google Scholar]