Abstract
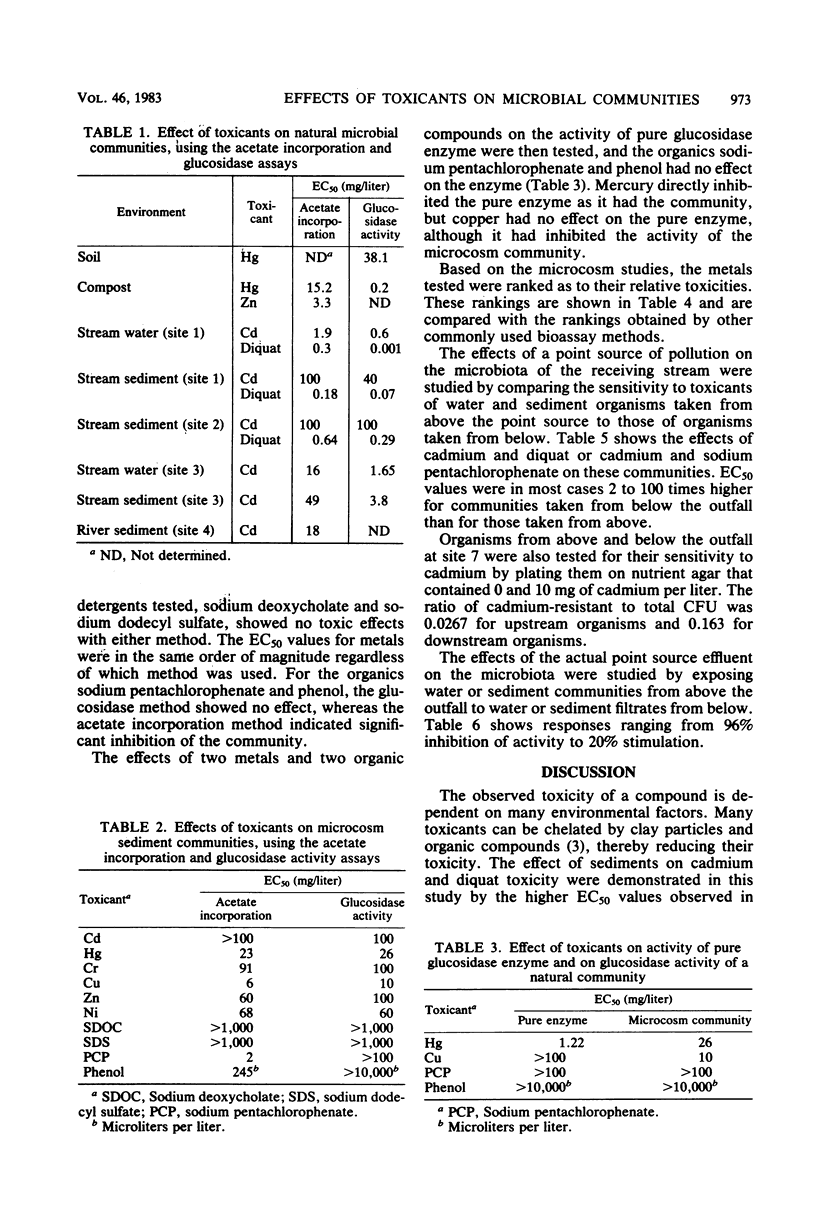
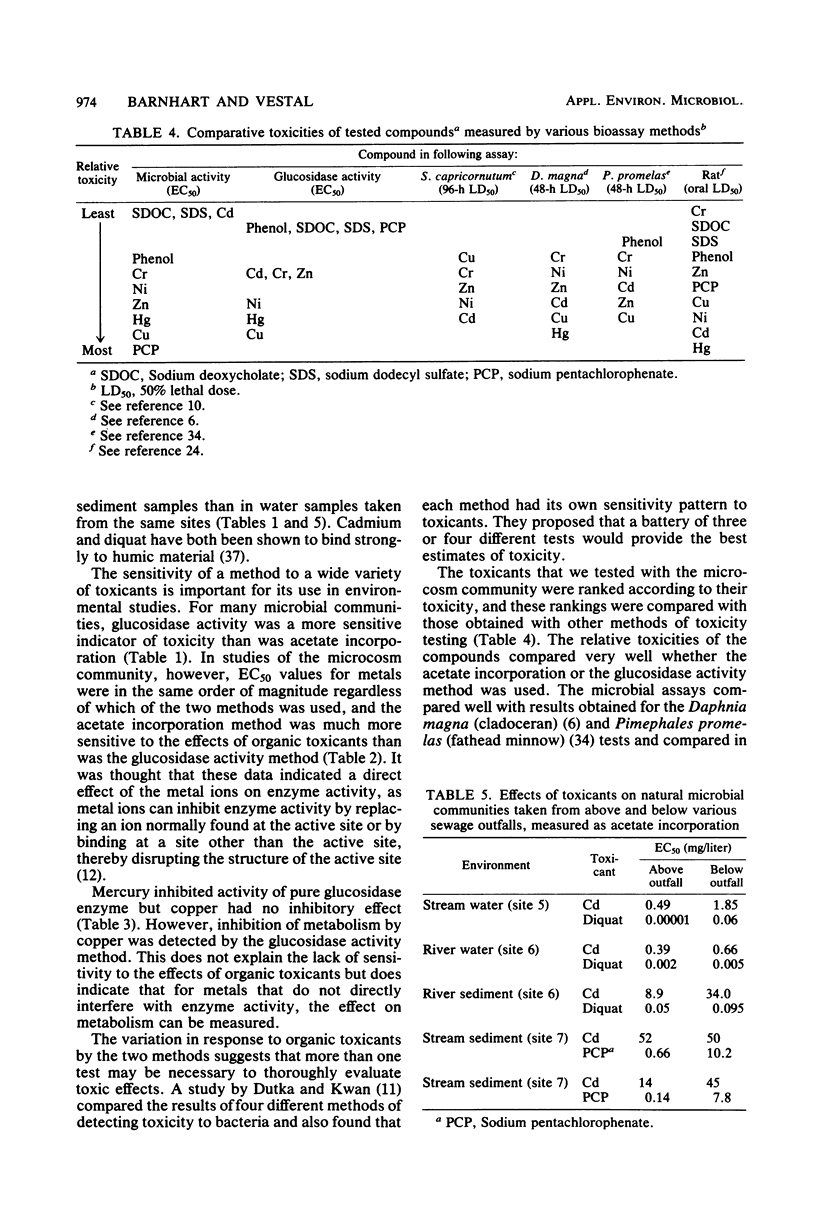
Two methods of measuring microbial activity were used to study the effects of toxicants on natural microbial communities. The methods were compared for suitability for toxicity testing, sensitivity, and adaptability to field applications. This study included measurements of the incorporation of 14C-labeled acetate into microbial lipids and microbial glucosidase activity. Activities were measured per unit biomass, determined as lipid phosphate. The effects of various organic and inorganic toxicants on various natural microbial communities were studied. Both methods were useful in detecting toxicity, and their comparative sensitivities varied with the system studied. In one system, the methods showed approximately the same sensitivities in testing the effects of metals, but the acetate incorporation method was more sensitive in detecting the toxicity of organic compounds. The incorporation method was used to study the effects of a point source of pollution on the microbiota of a receiving stream. Toxic doses were found to be two orders of magnitude higher in sediments than in water taken from the same site, indicating chelation or adsorption of the toxicant by the sediment. The microbiota taken from below a point source outfall was 2 to 100 times more resistant to the toxicants tested than was that taken from above the outfall. Downstream filtrates in most cases had an inhibitory effect on the natural microbiota taken from above the pollution source. The microbial methods were compared with commonly used bioassay methods, using higher organisms, and were found to be similar in ability to detect comparative toxicities of compounds, but were less sensitive than methods which use standard media because of the influences of environmental factors.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Babich H., Stotzky G. Environmental factors that influence the toxicity of heavy metal and gaseous pollutants to microorganisms. Crit Rev Microbiol. 1980;8(2):99–145. doi: 10.3109/10408418009081123. [DOI] [PubMed] [Google Scholar]
- Bewley R. J. Effects of heavy metal pollution on oak leaf microorganisms. Appl Environ Microbiol. 1980 Dec;40(6):1053–1059. doi: 10.1128/aem.40.6.1053-1059.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R. P., McNamara T. M., Caldwell B. A., Morita R. Y. Field observations on the acute effect of crude oil on glucose and glutamate uptake in samples collected from arctic and subarctic waters. Appl Environ Microbiol. 1981 Jun;41(6):1400–1406. doi: 10.1128/aem.41.6.1400-1406.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. B. Nitrogen fixation (acetylene reduction) in a salt marsh amended with sewage sludge and organic carbon and nitrogen compounds. Appl Environ Microbiol. 1977 Apr;33(4):846–852. doi: 10.1128/aem.33.4.846-852.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz A., Atlas R. M. Response of microorganisms to an accidental gasoline spillage in an arctic freshwater ecosystem. Appl Environ Microbiol. 1977 Jun;33(6):1252–1258. doi: 10.1128/aem.33.6.1252-1258.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEADBETTER E. R., FOSTER J. W. Studies on some methane-utilizing bacteria. Arch Mikrobiol. 1958;30(1):91–118. doi: 10.1007/BF00509229. [DOI] [PubMed] [Google Scholar]
- McKinley V. L., Federle T. W., Vestal J. R. Effects of petroleum hydrocarbons on plant litter microbiota in an arctic lake. Appl Environ Microbiol. 1982 Jan;43(1):129–135. doi: 10.1128/aem.43.1.129-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley V. L., Vestal J. R. Effects of Acid on plant litter decomposition in an arctic lake. Appl Environ Microbiol. 1982 May;43(5):1188–1195. doi: 10.1128/aem.43.5.1188-1195.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickels J. S., Bobbie R. J., Lott D. F., Martz R. F., Benson P. H., White D. C. Effect of manual brush cleaning on biomass and community structure of microfouling film formed on aluminum and titanium surfaces exposed to rapidly flowing seawater. Appl Environ Microbiol. 1981 Jun;41(6):1442–1453. doi: 10.1128/aem.41.6.1442-1453.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne L. L., Davies R. W., Ventullo R. M., Ladd T. I., Costerton J. W. The effects of chlorinated municipal sewage and temperature on the abundance of bacteria in the Sheep River, Alberta. Can J Microbiol. 1983 Feb;29(2):261–270. doi: 10.1139/m83-043. [DOI] [PubMed] [Google Scholar]
- Pickering Q. H., Henderson C. The acute toxicity of some heavy metals to different species of warmwater fishes. Air Water Pollut. 1966 Jun-Jul;10(6):453–463. [PubMed] [Google Scholar]
- Sayler G. S., Sherrill T. W., Perkins R. E., Mallory L. M., Shiaris M. P., Pedersen D. Impact of coal-coking effluent on sediment microbial communities: a multivariate approach. Appl Environ Microbiol. 1982 Nov;44(5):1118–1129. doi: 10.1128/aem.44.5.1118-1129.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildung R. E., Garland T. R. Effects of plutonium on soil microorganisms. Appl Environ Microbiol. 1982 Feb;43(2):418–423. doi: 10.1128/aem.43.2.418-423.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


