Abstract
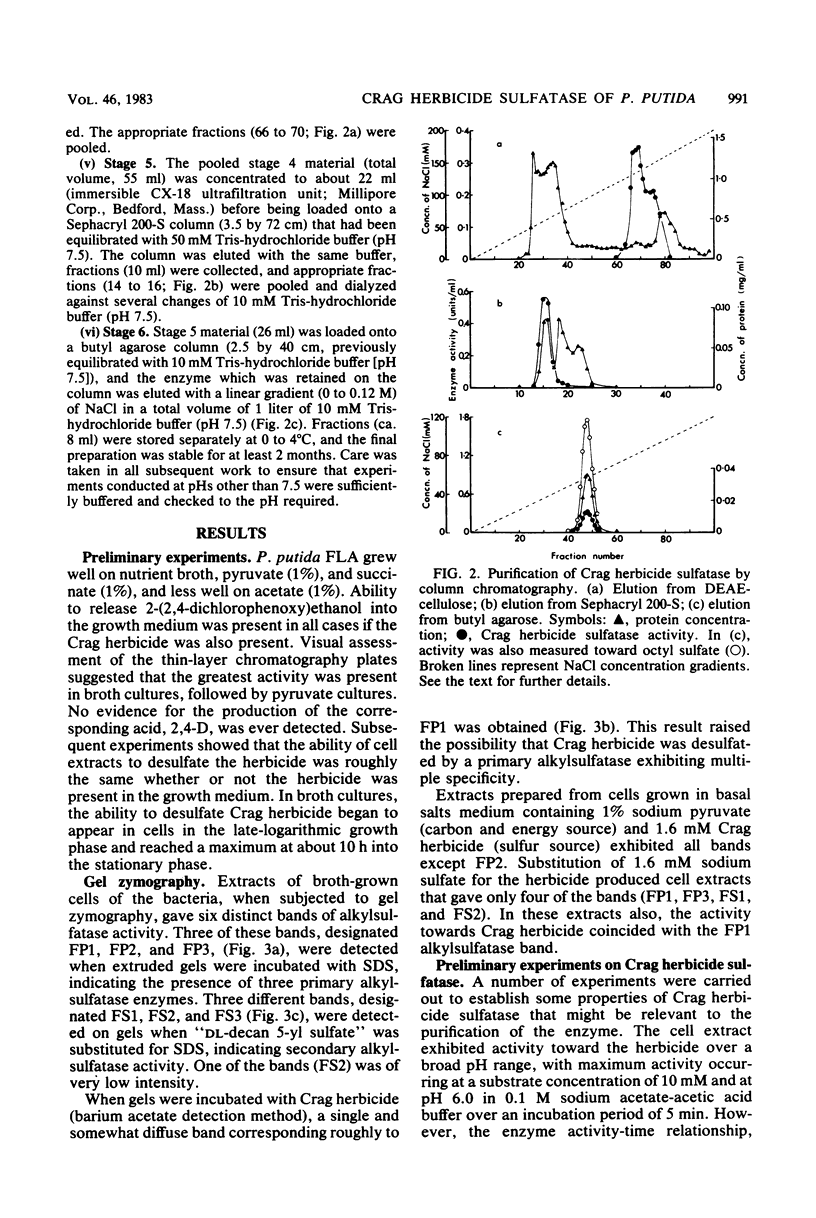
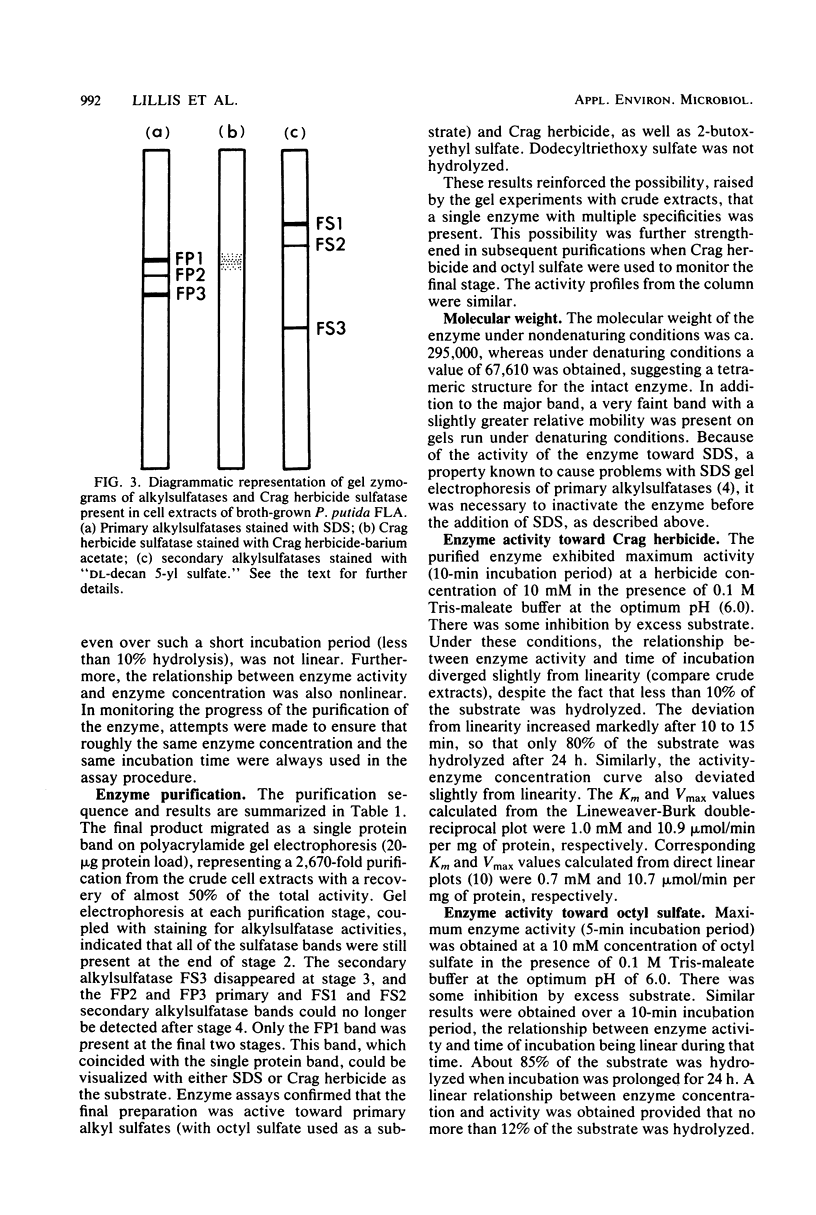
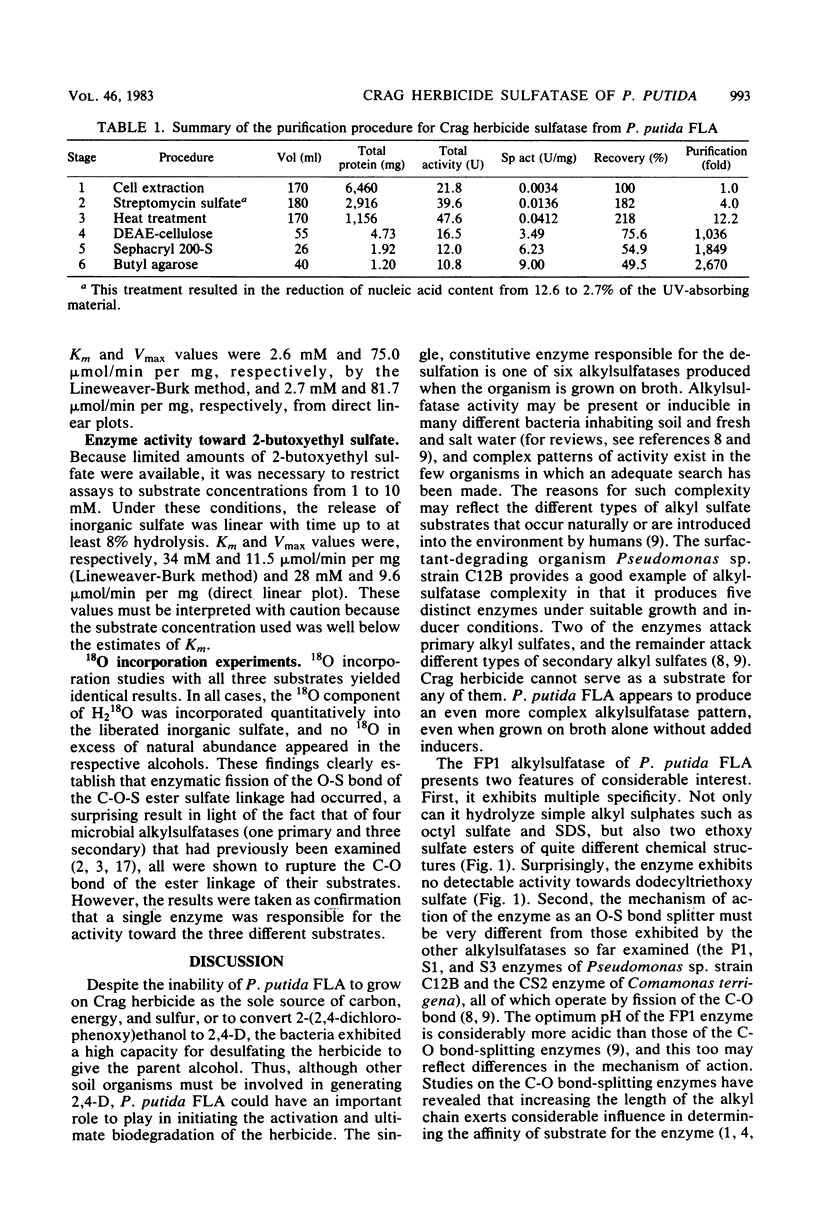
The activation of the preemergent herbicide 2-(2,4-dichlorophenoxy)ethyl sulfate (Crag herbicide) is initiated by soil microorganisms that are presumed to act by removing the ester sulfate group via some type of sulfatase enzyme. An enrichment technique with the herbicide as the sole source of sulfur led to the isolation of several pure cultures that could produce 2-(2,4-dichlorophenoxy)ethanol from the herbicide. One of these, a strain of Pseudomonas putida, was particularly active. Polyacrylamide gel zymograms of extracts of cells grown on nutrient broth showed the presence of three secondary and three primary alkylsulfatases. One of the latter enzymes was active toward Crag herbicide as well as sodium dodecyl sulfate. Maximum activity was obtained in the late-stationary phase of growth, and enzyme yields were not affected by either the presence or the absence of the herbicide in the growth medium. The enzyme was purified 2,670-fold to homogeneity by a combination of streptomycin sulfate treatment, heat treatment, and column chromatography on DEAE-cellulose, Sephacryl 200-S, and butyl agarose. The pure enzyme was tetrameric (molecular weight, 295,000) and most active at pH 6.0. Saturation kinetics with inhibition by excess substrate were observed for Crag herbicide and octyl sulfate. 2-Butox-yethyl sulfate was a relatively poor substrate, and dodecyltriethoxy sulfate was not hydrolyzed at all. Enzymatic hydrolysis of each substrate in the presence of H218O led to incorporation of 18O exclusively into SO42− ions in all three cases. The Crag herbicide sulfatase therefore acts by cleaving the O-S bond of the C-O-S ester linkage, in contrast with other alkylsulfatases acting on long-chain alkyl sulfates.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett C. H., Dodgson K. S., White G. F. Further studies on the substrate specificity and inhibition of the stereospecific CS2 secondary alkylsulphohydrolase of Comamonas terrigena. Biochem J. 1980 Nov 1;191(2):467–473. doi: 10.1042/bj1910467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew B., Dodgson K. S., Matcham G. W., Shaw D. J., White G. F. A novel mechanism of enzymic ester hydrolysis. Inversion of configuration and carbon-oxygen bond cleavage by secondary alkylsulphohydrolases from detergent-degrading micro-organisms. Biochem J. 1977 Sep 1;165(3):575–580. doi: 10.1042/bj1650575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloves J. M., Dodgson K. S., Games D. E., Shaw D. J., White G. F. The mechanism of action of primary alkylsulphohydrolase and arylsulphohydrolase from a detergent-degrading micro-organism. Biochem J. 1977 Dec 1;167(3):843–846. doi: 10.1042/bj1670843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloves J. M., Dodgson K. S., White G. F., Fitzgerald J. W. Purification and properties of the P2 primary alkylsulphohydrolase of the detergent-degrading bacterium pseudomonas C12B. Biochem J. 1980 Jan 1;185(1):23–31. doi: 10.1042/bj1850023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S. Determination of inorganic sulphate in studies on the enzymic and non-enzymic hydrolysis of carbohydrate and other sulphate esters. Biochem J. 1961 Feb;78:312–319. doi: 10.1042/bj0780312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diezel W., Kopperschläger G., Hofmann E. An improved procedure for protein staining in polyacrylamide gels with a new type of Coomassie Brilliant Blue. Anal Biochem. 1972 Aug;48(2):617–620. doi: 10.1016/0003-2697(72)90117-0. [DOI] [PubMed] [Google Scholar]
- Dodgson K. S., Fitzgerald J. W., Payne W. J. Chemically defined inducers of alkylsulphatases present in Pseudomonas C12B. Biochem J. 1974 Jan;138(1):53–62. doi: 10.1042/bj1380053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matcham G. W., Dodgson K. S. Preparation and characterization of substrates suitable for the study of stereospecific secondary alkylsulphohydrolases of detergent-degrading micro-organisms. Biochem J. 1977 Dec 1;167(3):717–722. doi: 10.1042/bj1670717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J., Fitzgerald J. W., Dodgson K. S. Methods for visualization of enzymes in polyacrylamide gels. Appl Microbiol. 1974 Jan;27(1):154–158. doi: 10.1128/am.27.1.154-158.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Shaw D. J., Dodgson K. S., White G. F. Substrate specificity and other properties of the inducible S3 secondary alkylsulphohydrolase purified from the detergent-degrading bacterium Pseudomonas C12B. Biochem J. 1980 Apr 1;187(1):181–190. doi: 10.1042/bj1870181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B. Studies on sulphatases. 25. The determination of BaSO(3)O by infrared spectroscopy. Biochem J. 1959 Nov;73(3):442–447. doi: 10.1042/bj0730442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. H., Tudball N. Studies on the enzymic degradation of L-serine O-sulphate by a rat liver preparation. Biochem J. 1967 Nov;105(2):467–472. doi: 10.1042/bj1050467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- White G. F., Lillis V., Shaw D. J. An improved procedure for the preparation of alkyl sulphate esters suitable for the study of secondary alkylsulphohydrolase enzymes. Biochem J. 1980 Apr 1;187(1):191–196. doi: 10.1042/bj1870191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaan J. Estimation of molecular weights of proteins by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Nov;21(2):155–168. doi: 10.1016/0003-2697(67)90177-7. [DOI] [PubMed] [Google Scholar]


