Abstract
Hantaviruses infect human endothelial cells and cause two vascular permeability-based diseases: hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. Hantavirus infection alone does not permeabilize endothelial cell monolayers. However, pathogenic hantaviruses inhibit the function of αvβ3 integrins on endothelial cells, and hemorrhagic disease and vascular permeability deficits are consequences of dysfunctional β3 integrins that normally regulate permeabilizing vascular endothelial growth factor (VEGF) responses. Here we show that pathogenic Hantaan, Andes, and New York-1 hantaviruses dramatically enhance the permeability of endothelial cells in response to VEGF, while the nonpathogenic hantaviruses Prospect Hill and Tula have no effect on endothelial cell permeability. Pathogenic hantaviruses directed endothelial cell permeability 2 to 3 days postinfection, coincident with pathogenic hantavirus inhibition of αvβ3 integrin functions, and hantavirus-directed permeability was inhibited by antibodies to VEGF receptor 2 (VEGFR2). These studies demonstrate that pathogenic hantaviruses, similar to αvβ3 integrin-deficient cells, specifically enhance VEGF-directed permeabilizing responses. Using the hantavirus permeability assay we further demonstrate that the endothelial-cell-specific growth factor angiopoietin 1 (Ang-1) and the platelet-derived lipid mediator sphingosine 1-phosphate (S1P) inhibit hantavirus directed endothelial cell permeability at physiologic concentrations. These results demonstrate the utility of a hantavirus permeability assay and rationalize the testing of Ang-1, S1P, and antibodies to VEGFR2 as potential hantavirus therapeutics. The central importance of β3 integrins and VEGF responses in vascular leak and hemorrhagic disease further suggest that altering β3 or VEGF responses may be a common feature of additional viral hemorrhagic diseases. As a result, our findings provide a potential mechanism for vascular leakage after infection by pathogenic hantaviruses and the means to inhibit hantavirus-directed endothelial cell permeability that may be applicable to additional vascular leak syndromes.
Hantaviruses cause two highly lethal diseases: hemorrhagic fever with renal syndrome (HFRS) and hantavirus pulmonary syndrome (HPS) (64, 65). Both diseases are manifestations of enhanced vascular permeability leading to hemorrhage or acute pulmonary edema in patients days to weeks after infection (34, 49, 65, 93). Acute thrombocytopenia is common to both syndromes, and acute disease is associated with cardiopulmonary dysfunction, high viral load, and the severity of platelet loss (11, 39, 53, 90). As a result, both endothelial barrier functions and platelet directed responses that restore vascular barrier functions are compromised following hantavirus infection (49, 64, 93).
Hantaviruses predominantly infect endothelial cells that form a fluid barrier within the vasculature and perform central roles in vascular repair (49, 93). If hantaviruses lysed endothelial cells, the mechanism of hantavirus directed vascular permeability would be clear and result in hemorrhagic disease. However, hantaviruses are not lytic, and endothelial cell monolayers are not permeabilized by hantavirus infection alone (8, 35, 49, 65, 72, 93). The absence of hantavirus permeability assays, limited animal models of hantavirus disease, and biosafety level 4 requirements for investigating hantavirus disease in Syrian hamsters (27, 37, 84) has further limited our ability to study hantavirus pathogenesis. As a result, the mechanism of hantavirus-directed permeability and disease remains undefined but directly tied to infection of endothelial cells.
Suggestions for the causes of hantavirus permeability are abundant but remain untested and reflect responses that are common to many viruses that lack vascular disease correlates. T cells, tumor necrosis factor alpha (TNF-α), and cytokines have been suggested to be involved in permeability, but their contribution to HFRS or HPS diseases remains a question (23, 35, 76). A prominent link between vascular permeability disorders and hantavirus disease stems from the role of β3 integrins in hemorrhagic disease and the use and dysregulation of β3 integrin function by pathogenic hantaviruses (3, 15, 19-21, 25). Mutations in β3 integrins result in Glanzmann thrombasthenia, a human genetic hemorrhagic disease, and autoimmune antibodies to β3 similarly direct hemorrhagic disease (15, 25, 29, 32, 58, 87). Knocking out β3 integrins in mice mimics the microvascular permeability and hemorrhage of Glanzmann thrombasthenia and demonstrates the role of β3 integrins in maintaining vascular integrity (15, 25, 29). Interestingly, pathogenic, but not nonpathogenic, hantaviruses reportedly bind and dysregulate the function of αvβ3 integrins on human endothelial cells (19-21). HFRS and HPS causing hantaviruses block αvβ3-directed endothelial cell migration 2 to 4 days after infection and thereby inhibit αvβ3 integrin function days after viral adsorption (19-21, 54). A mechanism for hantavirus inhibition of αvβ3 function is provided by findings demonstrating that pathogenic hantaviruses interact with plexin-semaphorin-integrin domains present at the apex of bent, inactive conformations of αvβ3 (54, 73). Since antibodies to β3 or the absence of β3 function result in vascular permeability disorders, the dysregulation of β3 integrin function by pathogenic hantaviruses provides a compelling mechanism for vascular permeability defects following hantavirus infection (15, 25, 58).
β3 integrins regulate vascular permeability through effects on vascular endothelial growth factor (VEGF) (12, 55, 57). αvβ3 works coordinately with VEGF to direct endothelial cell migration during angiogenesis and to reseal the endothelium in response to damage (9, 29, 57, 81). Migration itself is a complex series of events that requires VEGF-directed loosening of tightly adherent endothelial cells in order to permit movement, while at the same time endothelial cell adherence to integrins provides a vector to cell movement (56). Endothelial cell migration requires VEGF, and VEGF was initially called vascular permeability factor for observations that it potently directed edema within tissues (12, 48, 68). In fact, VEGF is reportedly 50,000 times more potent than histamine in inducing vascular permeability (12). Interestingly, β3−/− mice and endothelial cell monolayers are sensitized to the effects of VEGF and hyperpermeable in response to VEGF addition (12, 55, 57). These findings indicate that αvβ3 modulates vascular permeability responses directed by VEGF and regulates capillary leakage (57).
Endothelial cell permeability is regulated by additional factors derived from platelets and plasma (2, 10, 30, 42-44, 51, 63). Angiopoietin 1 (Ang-1) is an endothelial-cell-specific growth factor that counters the permeabilizing effects of VEGF and stabilizes the vasculature (41, 77, 78). Ang-1 binding to Tie-2 receptors reduces endothelial cell permeability and has a dominant effect over the permeabilizing responses of coadministered VEGF (16, 31, 78). Platelets are also an abundant source of the endothelial cell permeability regulator sphingosine 1-phosphate (S1P) (30, 44, 63, 75). Activated platelets release S1P, which binds to G-protein-coupled Edg-1 receptors on endothelial cells and stabilizes intracellular junctions (44, 63, 74, 91).
Here we show that pathogenic HFRS and HPS causing hantaviruses sensitize human endothelial cells to the permeabilizing effects of VEGF. Hyperpermeability of endothelial cell monolayers occurs 2 to 3 days after infection and is inhibited by Ang-1, S1P, and antibodies to VEGF receptor 2 (VEGFR2). In contrast, the nonpathogenic hantaviruses Prospect Hill virus (PHV) and Tula virus (TULV) had no effect on endothelial cell permeability in the presence or absence of VEGF. The enhanced VEGF permeability of endothelial cells infected with pathogenic hantaviruses mimics the VEGF-directed permeability responses of β3 integrin knockouts (25, 57), and correlates directly with β3 integrin dysfunction. These findings provide a hantavirus permeability assay which has identified Ang-1, S1P, or antibodies to VEGFR2 as inhibitors of hantavirus-directed endothelial cell permeability. Since these factors are already in clinical trials for other indications, potential therapeutics may already be available for hantavirus patients (7, 42, 46, 59, 66), and it is possible that these factors could regulate capillary permeability caused by additional hemorrhagic viruses. Our findings provide rationales for the testing of vascular permeability inhibitors in the Syrian hamster model of hantavirus disease (27) and further suggest that VEGF-, Ang-1-, and S1P-directed signaling responses may provide additional therapeutic targets for inhibiting hantavirus directed permeability.
MATERIALS AND METHODS
Cells and virus.
BSL-3 facilities were used for Hantaan virus (HTNV), Andes virus (ANDV), and NY-1 virus (NY-1V) cultivation (18, 19, 21). Human umbilical vein endothelial cells (HUVECs, passage 3-7) were purchased from Cambrex and grown in supplemented EBM-2 medium (Cambrex). HTNV (76-118), NY-1V, ANDV (CHI-7913), PHV, and TULV (Tula/Moravia/MA 5302V/94) (80) were cultivated on Vero E6 cells as previously described (19). Virus titers were determined by focus assay after immunoperoxidase staining of hantavirus nucleocapsid protein within cells as previously described (21).
Ligand and antibodies.
Vitronectin was obtained from Chemicon, VEGF165 and fluorescein isothiocyanate (FITC)-dextran 40,000 were from Sigma. Ang-1 (human) was obtained from Alexis Biochemicals, and S1P was from Avanti-Polar Lipids. Antibody to Flk-1 (clone 89106) was from R&D Systems, and rabbit polyclonal antibody to Edg1 (antibody 13126) was obtained from Abcam. Normal mouse immunoglobulin G was from Santa Cruz Biotechnology. Goat anti-rabbit horseradish peroxidase conjugates were obtained from Amersham.
Endothelial cell permeability assay.
HUVECs were seeded onto vitronectin (10 μg/ml)-coated Costar Transwell inserts (6.5-mm diameter and 3-μm pore size; Corning) at a cell density of 2 × 104 cells and grown in EBM-2 with 10% of fetal calf serum. Confluent HUVECs were infected in triplicate with pathogenic HTNV, ANDV, or NY-1V or nonpathogenic PHV or TULV at a multiplicity of infection (MOI) of 0.5 or mock infected. After 1 h of adsorption or 1, 2, or 3 days postinfection, cells were starved overnight with EBM-2-0.5% bovine serum albumin without growth factors. FITC-dextran (0.5 mg/ml) was added to the upper chamber of monolayers in the presence or absence of VEGF (100 ng/ml) as previously described (79). At various times, FITC-dextran present in the lower chamber was assayed by using a Perkin-Elmer fluorimeter (490-nm excitation, 530-nm emission), and fluorescence intensity measurements were expressed directly or as the fold increase over the basal permeability of monolayers. In order to monitor hantavirus infections, endothelial cell monolayers in 96-well plates were infected at the same MOI as the Transwell plates, and at 24 or 72 h postinfection the cells were fixed and the hantavirus N-protein present in cells was detected by immunoperoxidase staining (21).
Inhibition of hantavirus-induced HUVEC permeability.
HUVECs were grown in Transwell plates and infected as described above. FITC-dextran and VEGF were added to the upper chamber of cells in the presence or absence of Ang-1 (50 ng/ml) or S1P (0.01 to 1 μM) at 3 days postinfection. The permeability of monolayers was evaluated by quantitating the appearance of FITC-dextran in the lower chamber as described above. S1P was solubilized in methanol and stored at −20°C in 0.4% (vol/vol) methanol. An identical amount of methanol diluent was added to EBM-2 medium (0.5% bovine serum albumin) in S1P control experiments. Antibodies to VEGFR2 (100 ng/ml) or the S1P receptor, Edg1 (100 ng/ml), were added to infected or mock-infected cells for 30 min at 37°C prior to the addition of FITC-dextran, and permeability assays were performed as described above. The results are derived from two to five independent experiments (n = 9, n = 15) with a P value of <0.05. The results are reported as the mean ± the standard deviation of the fold permeability for each group. A two-tailed Student t test was used to analyze statistical differences between control and treated groups. Differences were considered statistically significant at P < 0.05.
RESULTS
Hantavirus infection enhances the permeabilizing effects of VEGF.
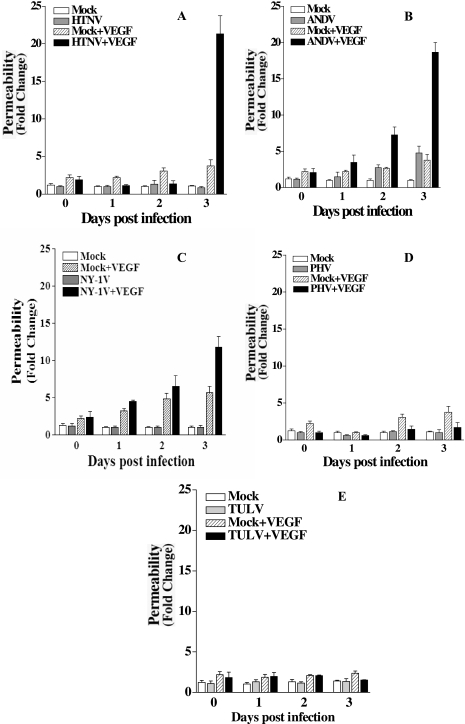
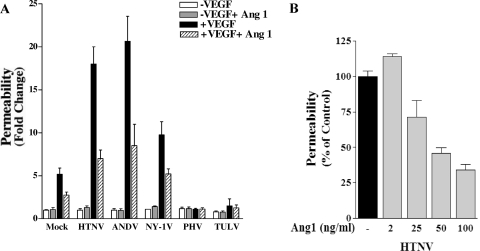
Hantaviruses predominantly infect human endothelial cells, and only pathogenic hantaviruses bind inactive bent conformations of αvβ3 integrins on endothelial cells (19, 21, 54, 73). Pathogenic hantaviruses block the function of αvβ3 integrins on endothelial cells, and αvβ3 normally regulates the permeabilizing effects of VEGF (20, 57). These findings suggested that pathogenic hantavirus dysregulation of αvβ3 integrin function might enhance endothelial cell permeability and contribute to hantavirus pathogenesis. In order to study hantavirus permeability, we used a standard cell permeability assay (22, 79), which monitors the passage of FITC dextran across monolayers and evaluated the effect of VEGF on hantavirus-infected endothelial cells. HUVECs were plated on Transwell plates, and confluent monolayers were infected with pathogenic HTNV, ANDV, NY-1V, or nonpathogenic PHV or TULV. Monolayer permeability was assayed by adding FITC-dextran to the upper chamber in the presence or absence of VEGF and evaluating the emigration of the FITC-dextran to the lower chamber by fluorimetry. As previously reported (35), hantavirus infection alone did not permeabilize endothelial cell monolayers regardless of whether endothelial cells were infected with pathogenic or nonpathogenic hantaviruses (Fig. 1 and 2). In addition, infection of endothelial cells with nonpathogenic PHV or TULV did not permeabilize endothelial cells in the presence or absence of VEGF (Fig. 1D and E and Fig. 2G to J). In contrast, HTNV, ANDV, and NY-1V dramatically increased endothelial cell permeability 18- to 23-fold in response to VEGF 3 days postinfection (Fig. 1A to C). ANDV also enhanced endothelial cell permeability 2 days postinfection (Fig. 1B), whereas the endothelial cell permeability responses to VEGF were the same as those to mock-infected controls 1 day postinfection. These findings indicate that pathogenic hantaviruses substantially enhanced the permeability of endothelial cells in response to VEGF 3 days postinfection and provided a hantavirus permeability assay for further studies.
FIG. 1.
Pathogenic hantaviruses enhance endothelial cell permeability. Endothelial cells were plated on vitronectin-coated Transwell inserts and infected at an MOI of 0.5 in triplicate with HTNV (A), ANDV (B), NY-1V (C), PHV (D), or TULV (E) or mock infected. FITC-dextran was added to upper chamber media in the presence or absence of VEGF, and the presence of FITC dextran in the lower chamber was quantitated after 3 h (79). The results are expressed as the fold increase in monolayer permeability over basal permeability levels.
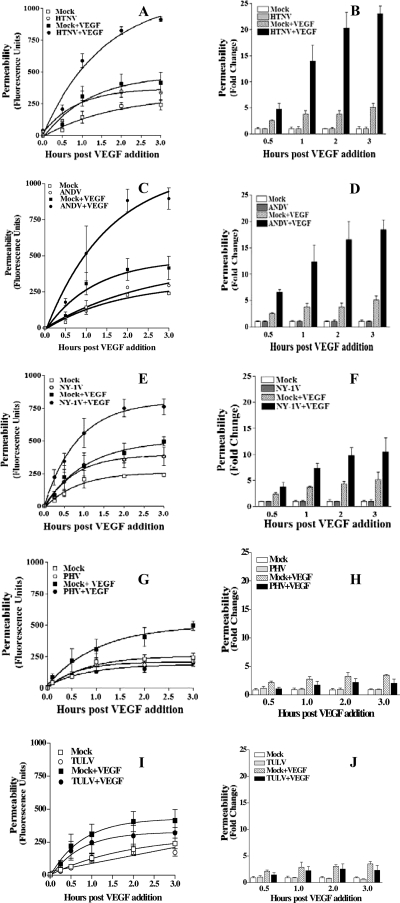
FIG. 2.
Time dependence of hantavirus enhanced permeability. Hantavirus-directed permeability assays were performed as in Fig. 1 at 3 days postinfection with HTNV (A and B), ANDV (C and D), NY-1V (E and F), PHV (G and H), or TULV (I and J). FITC-dextran in the lower chamber was measured at the indicated times after VEGF addition and compared to similarly treated mock-infected cells. Primary fluorescence data for HTNV, ANDV, NY-1V, PHV, and TULV are presented (A, C, E, G, and I), and the results are presented as the fold change in basal permeability at the same time after treatment (B, D, F, H, and J).
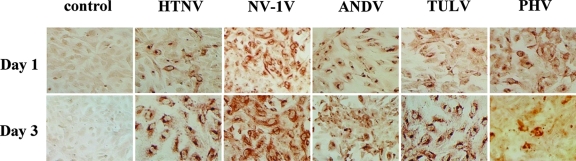
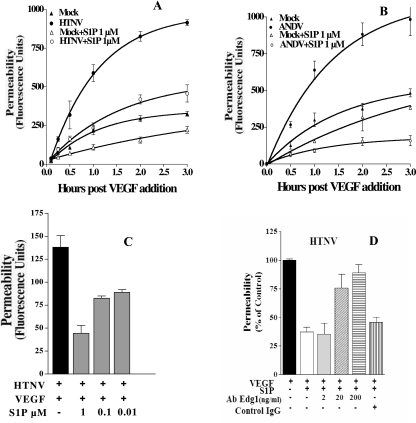
In order to investigate the responsiveness of hantavirus-infected endothelial cells to VEGF, we analyzed endothelial cell permeability at various times after VEGF addition. The permeability of HTNV-, ANDV-, and NY-1V-infected endothelial cells increased dramatically from 1 to 3 h after VEGF addition (Fig. 2A to F). Even 0.5 h postinfection, ∼2-fold increases in permeability were observed in cells infected by pathogenic hantaviruses compared to VEGF-treated mock-infected cells. HTNV infection resulted in the largest increase in endothelial cell monolayer permeability of any virus tested (Fig. 1A and Fig. 2A and B). In contrast PHV and TULV infection did not enhance VEGF permeability responses at any time and were virtually identical to mock-infected controls (Fig. 2G to J). PHV and TULV infection actually reduced VEGF-directed permeability below the basal responses of mock-infected cells (Fig. 2G to J). Even though a constant MOI was used to infect cells, we comparatively evaluated nucleocapsid protein expression within endothelial cells 1 and 3 days postinfection. Figure 3 shows that endothelial cells were comparably infected by pathogenic and nonpathogenic hantaviruses 1 and 3 days postinfection. There is a reduction in the number of PHV-infected cells 3 days postinfection as previously reported (1). In addition, virtually all cells are infected with the nonpathogenic TULV 3 days postinfection, similar to pathogenic hantaviruses, and yet TULV infection does not enhance endothelial cell permeability in response to VEGF (Fig. 2I to J). These findings demonstrate the time dependence of hantavirus-enhanced permeability responses and indicate that only pathogenic hantaviruses enhance endothelial cell permeability in response to VEGF.
FIG. 3.
Hantavirus infection of endothelial cells. HUVECs were plated on microtiter plates and infected with the indicated hantaviruses at an MOI of 0.5 or were mock infected. Cells were fixed 1 or 3 days postinfection, and hantavirus nucleocapsid protein within infected cells was detected by using a previously described immunoperoxidase staining approach (21).
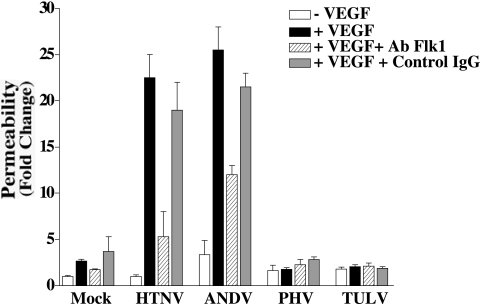
Effect of antibodies to Flk-1 on hantavirus-directed endothelial cell permeability.
Endothelial cells contain VEGFR2 receptors (Flk-1), which mediate migrational responses of endothelial cells to VEGF (50). To further demonstrate the specificity of enhanced permeability responses after hantavirus infection, we determined whether antibodies to VEGFR2 inhibited permeabilizing endothelial cell responses. Hantavirus-infected or mock-infected HUVECs were pretreated with antibodies to VEGFR2 or an isotype-matched control antibody, and the permeability of the endothelial cells was evaluated in the presence or absence of VEGF. Figure 4 indicates that antibodies to VEGFR2 reduced the permeability of HTNV- and ANDV-infected cells by 50 to 75%, while there was no change in endothelial cell permeability after control antibody pretreatment or PHV or TULV infection. These findings suggest that pathogenic hantavirus-directed endothelial cell permeability is at least partly dependent on the function of VEGFR2.
FIG. 4.
Effect of antibody to VEGFR2 on hantavirus-directed EC permeability. HUVECs were grown as in Fig. 1 and infected at an MOI of 0.5 or mock infected. At 3 days postinfection, monoclonal antibody to VEGFR2 (100 ng/ml) or an isotype-matched control (mouse IgG, 100 ng/ml) was added to cells for 30 min at 37°C. The antibodies were removed, and the permeability of infected and mock-infected cells was evaluated as in Fig. 1. Error bars represent the mean ± the standard deviation (n = 9 from three independent experiments).
Hantavirus-directed endothelial cell permeability is inhibited by Ang-1.
Ang-1 is an endothelial-cell-specific growth factor that binds to a unique growth factor receptor, Tie-2 (14). However, unlike VEGF, Ang-1 enhances endothelial cell barrier function and inhibits endothelial cell permeability (16, 77, 78). In order to determine whether Ang-1 blocks hantavirus-enhanced endothelial cell permeability, we assayed the permeability of hantavirus-infected endothelial cells in the presence or absence of Ang-1. Figure 5A indicates that the addition of Ang-1 to hantavirus-infected endothelial cells resulted in a 50 to 60% reduction in endothelial cell permeability, and Fig. 5B demonstrates that Ang-1 inhibition of hantavirus-directed endothelial cell permeability is dose dependent. These findings indicate that Ang-1 inhibits hantavirus-directed endothelial cell permeability and suggest the potential for Ang-1 to regulate vascular permeability defects within HPS and HFRS patients.
FIG. 5.
Ang-1 inhibition of hantavirus-directed EC permeability. (A) HUVECs were infected as in Fig. 1. At 3 days postinfection FITC-dextran and VEGF were added to the upper chamber in the presence or absence of Ang-1 (50 ng/ml), and FITC-dextran in the lower chamber was quantitated 3 h later. (B) Cells were infected, treated, and monitored as described above in the presence of increasing amounts of Ang-1 added to the upper chamber. The results are derived from two independent experiments (n = 6).
S1P inhibits hantavirus-directed endothelial cell permeability.
Hantaviruses cause acute thrombocytopenia, and the magnitude of platelet reduction is associated with enhanced pulmonary edema in HPS patients (93). Platelets store abundant amounts of S1P, which is a potent endothelial barrier enhancing factor (43, 44, 63). S1P is released by activated platelets and decreases vascular leakage by binding to Edg-1 receptors on endothelial cells (63, 74). Since S1P enhances endothelial barrier functions, we determined whether S1P was capable of inhibiting hantavirus-directed endothelial cell permeability. The permeability of mock-infected or HTNV-infected endothelial cells was evaluated 3 days postinfection in the presence or absence of physiologic concentrations of S1P. S1P inhibited HTNV- and ANDV-directed endothelial cell permeability from 0.5 to 3 h after addition and was dose dependent (Fig. 6A to C). Pretreatment of cells with an increasing amount of a function blocking antibody to the S1P receptor, Edg-1, prevented the inhibitory effects of S1P and resulted in a corresponding increase in hantavirus-directed permeability (Fig. 6D). These findings indicate that the addition of S1P inhibits hantavirus-directed endothelial cell permeability and that S1P-directed effects are mediated by the Edg-1 receptor on endothelial cells. These findings suggest a second potential therapeutic approach for abrogating vascular permeabilizing effects of pathogenic hantaviruses.
FIG. 6.
S1P inhibits hantavirus-directed EC permeability. HUVECs were infected with HTNV (A) or ANDV (B), and the endothelial cell monolayer permeability was assessed at the indicated times, as in Fig. 1, in the presence or absence of S1P (1 μM) (43). (C) Cells were infected and treated as described above in the presence of increasing amounts of S1P added to the upper chamber. Monolayer permeability was analyzed as described above, and the results are derived from two independent experiments, performed in triplicate. (D) HUVEC permeability in response to HTNV infection was determined as described above in the presence or absence of antibodies to the S1P receptor, Edg1, or an isotype-matched control antibody. After removal of the antibodies, cells were treated with FITC-dextran and VEGF in the presence or absence of S1P (1 μM). The permeability of endothelial cells was assessed by quantitating the FITC-dextran in the lower chamber 3 h after VEGF addition.
DISCUSSION
Pathogenic hantaviruses inhibit the function of β3 integrins, and both hantaviruses and β3 integrins are intimately tied to hemorrhagic disease and vascular permeability disorders (15, 19-21, 25, 29). In fact, β3 integrin knockout mice mimic the microvascular permeability and platelet dysfunction of Glanzmann's disease, and both β3 dysregulation and thrombocytopenia are common features of HPS- and HFRS-causing viruses (25, 29, 57). The dependence of αvβ3-directed migration on VEGF cues is well known; however, the absence of αvβ3 also results in the hyperpermeability of endothelial cells in response to VEGF, and thus VEGF-directed permeabilizing responses are normally regulated by αvβ3 (25, 29, 55, 57). The link between β3 dysfunction and enhanced VEGF-directed vascular permeability suggested that a similar scenario might direct permeabilizing responses of pathogenic hantaviruses that block the function of β3 integrins days after infection (20). Findings presented here demonstrate that both HFRS- and HPS-causing hantaviruses direct the hyperpermeability of infected endothelial cells in response to VEGF, and these findings suggest a means by which hantavirus dysregulation of β3 integrin function enhances permeabilizing VEGF responses that may contribute to hantavirus disease.
Suggestions for causes of hantavirus permeability are abundant, and T cells and TNF-α have been suggested to be involved in hantavirus-associated disease (35, 76). Primed cytotoxic T lymphocytes kill infected endothelial cells in vitro, which has been suggested as a mechanism of capillary permeability (23). However, the endothelium of HPS patients is not damaged, and cytotoxic-T-lymphocyte-directed lysis of endothelial cells would likely result in hemorrhagic disease rather than the transudative pulmonary edema observed during HPS (8, 76). In addition, TNF-α does not increase the permeability of hantavirus-infected endothelial cells in vitro (35), and in HFRS patients an increase in soluble TNF receptors is suggested to limit TNF-α availability (40). Pulmonary fluid accumulation during HPS has been reported to be primarily or exclusively transudative (8), further suggesting that regulators of endothelial cell permeability and edema, such as VEGF, may play a primary role in hantavirus pathogenesis (8, 9, 24, 33, 45, 47, 52, 88). However, pathogenesis is a systemic response, and the role of additional immune cell, chemokine, or cytokine responses in hantavirus-directed endothelial cell permeability cannot be excluded (40).
Acute thrombocytopenia and vascular permeability deficits are hallmarks of HFRS and HPS diseases (49, 64, 93). Pathogenic hantaviruses predominantly infect endothelial cells, and resulting hantavirus diseases are symptomatic of vascular permeability deficits within the microvasculature (49, 93). Endothelial cells and platelets are central regulators of vascular permeability, and β3 integrins are key determinants of endothelial cell and platelet function that stabilize the vasculature (25, 29, 43, 44, 68). Pathogenic hantaviruses block the migration of infected endothelial cells on vitronectin, the high-affinity αvβ3 integrin ligand, but have no effect on migration directed by other integrins, suggesting that this effect is not a result of inhibiting common downstream integrin signaling pathways or the formation of focal adhesions (20). Pathogenic hantaviruses do not appear to alter the amount of αvβ3 receptors on the surface of endothelial cells, and the selective dysregulation of αvβ3 is consistent with the reported interactions of pathogenic hantaviruses with inactive, bent αvβ3 integrin conformers days after infection (20, 28, 54, 73). Like β3 integrin knockouts (57), endothelial cells are not permeabilized by hantavirus infection alone (35) but, in response to VEGF, pathogenic hantavirus-infected endothelial cells are highly permeabilized even in the absence of capillary pressure. These findings tie the dysregulation of β3 integrins by pathogenic hantaviruses to the VEGF hyper-responsiveness of endothelial cells infected by pathogenic hantaviruses.
Knowing the central importance of VEGF in regulating vascular permeability, the role of VEGF in hantavirus-directed endothelial cell permeability is not surprising (4, 26, 47, 57, 60, 82, 83, 88, 92). VEGF is reportedly 50,000 times more potent than histamine in inducing vascular permeability, and VEGF was originally named vascular permeability factor as a result of its ability to induce edema within tissues (12, 17, 68). VEGF is a chemokine that promotes the extravasation of immune cells, and VEGF acts directly on endothelial cells by binding to VEGFR2 growth factor receptors which direct a number of intracellular signaling responses through its cytoplasmic tyrosine kinase domain (50). VEGF also binds to neuropilin-1 (NP-1) on endothelial cells, and NP-1 coreceptors form complexes with VEGFR2 that enhance the permeabilizing effects of VEGF-A isoforms (5). αvβ3 is also reported to interact with VEGFR2 (69), although the means by which NP-1, VEGFR2, and αvβ3 coordinately regulate endothelial cell signaling and permeabilizing responses are complex, and the mechanism of αvβ3 integrin regulation of VEGF directed permeability has not been defined.
Ang-1, like VEGF, is a second endothelial-cell-specific growth factor, and Ang-1 binds Tie-2 receptors on endothelial cells to elicit growth-factor-directed endothelial cell responses (38). In contrast to VEGF, Ang-1 does not enhance vascular permeability but instead stabilizes capillaries, directs the assembly of adherens junctions, and promotes vascular barrier functions (16, 41, 77, 78, 85). In fact, Ang-1-directed vascular stability is dominant to the permeabilizing effects of VEGF, since mice that are cotransgenic for both Ang-1 and VEGF have the heightened vascular barrier phenotype of Ang-1 transgenic animals (78). The regulatory role of Ang-1 in stabilizing the vasculature prompted us to determine whether hantavirus-directed permeability of endothelial cells was similarly Ang-1 regulated. Our data indicates that Ang-1 blocks endothelial cell permeability directed by pathogenic hantaviruses, and these findings provide a rationale for studies of Ang-1 as an inhibitor of the vascular permeability deficits within the Syrian hamster model of HPS, as well as hantavirus patients.
Hantavirus patients present with severe disease days to weeks after their initial hantavirus exposure, and acute thrombocytopenia is a common element of HPS and HFRS disease (49, 64, 93). Although there is no understanding of hantavirus interactions with platelets, β3 integrins are the most abundant receptors present on platelets (71). Thrombocytopenia is likely to contribute to vascular barrier deficits during hantavirus infection, since platelets play clear roles in regulating vascular permeability. Aside from the role of platelets in clotting cascades, platelets also contribute to endothelial cell barrier function by releasing the lipid mediator S1P, which binds to G-protein-coupled Edg-1 receptors on endothelial cells (30, 43, 63). S1P enhances the accumulation of vascular-endothelial cadherin (VE-cadherin), which uniquely ties endothelial cells to each other and stabilizes adherens junctions (44, 85, 91). In contrast, VEGFR2 activation directs the dissociation of VE-cadherins, which enhances vascular permeability, and thus VEGF and S1P direct opposing vascular responses (16, 17, 85, 91). The acute loss of platelets during hantavirus infection is another potential point where endothelial cell permeability regulators, such as S1P, may be diminished during hantavirus infection and contribute to vascular permeability. Our findings demonstrate that S1P inhibits hantavirus-directed endothelial cell permeability and suggest that a platelet-derived factor has the potential to reduce vascular permeability deficits within hantavirus patients.
Thrombocytopenia normally sets in motion a series of events that repair the endothelium and replenish circulating platelets (36, 75). Stromal cells sense thrombocytopenia and respond by producing thrombopoietin (TPO) (36). TPO induces the production of VEGF, and the combination of VEGF and TPO directs the growth and differentiation of megakaryocytes, resulting in an increase in circulating platelets (6, 36, 75). VEGF disrupts endothelial cell adherens junctions (17, 86), enhancing permeability to permit endothelial cell movement, while αvβ3 and S1P limit the permeabilizing effects of VEGF by directing VE-cadherin assembly between endothelial cells (43, 44, 67, 85, 86, 91). One possibility is that in the context of sustained thrombocytopenia and hantavirus dysregulation of αvβ3 function, permeabilizing VEGF responses may continue in the absence of compensating αvβ3 or S1P regulatory responses.
The data presented here indicate that S1P, Ang-1, and antibodies to VEGFR2 block hantavirus-directed endothelial cell permeability, and as a result these factors are potential therapeutics for hantavirus diseases. Our data further suggest that pathways downstream of the Edg-1, Tie-2, and VEGFR2 receptors or which direct the assembly of adherens junctions may be additional targets for regulating hantavirus directed permeability. Since S1P, FTY720-P (an S1P analog), platelets, Ang-1, and antibodies to VEGF or VEGFR2 are used clinically for other indications (7, 42, 46, 59, 66), therapeutics for hantavirus patients may already be viable clinical options.
Although there is no information on what differentiates hantaviruses that cause hemorrhagic disease (HFRS) from hantaviruses that cause acute pulmonary edema (HPS), either disease may have pulmonary or renal manifestations, and capillary beds are abundant in both tissues (13, 49, 64, 93). Interestingly, VEGF also directs glomerular endothelial cell permeability (62), and thus permeability changes within the kidney during hantavirus renal syndrome could similarly involve dysregulated endothelial cell responses to VEGF (60-62, 89). Permeabilizing endothelial cell responses to VEGF could also contribute to additional viral hemorrhagic diseases. Dengue virus causes hemorrhagic disease with acute thrombocytopenia. Recent reports indicate that dengue viruses use β3 integrins, that VEGF levels are enhanced in dengue patients, and that soluble VEGFR-2 levels were inversely correlated the severity of plasma leakage in patients (70, 94). These findings suggest that the effects of VEGF may be a common factor in the vascular leakage directed by pathogenic viruses. The broad function of Ang-1, S1P, and anti-VEGFR2 antibodies in regulating vascular permeability suggests that these factors may similarly inhibit vascular permeability resulting from other viral hemorrhagic agents.
Acknowledgments
We are grateful to O. Vapalahti for providing the TULV used in this study. We thank Varya Kirillov for technical assistance and Adrish Sen and Nandini Sen for critical comments.
This study was supported by National Institutes of Health grants R01AI47873, PO1AI055621, and U54AI57158 (Northeast Biodefense Center-Lipkin) and by a Veterans Affairs Merit Award.
Footnotes
Published ahead of print on 26 March 2008.
REFERENCES
- 1.Alff, P. J., I. N. Gavrilovskaya, E. Gorbunova, K. Endriss, Y. Chong, E. Geimonen, N. Sen, N. C. Reich, and E. R. Mackow. 2006. The pathogenic N.Y.-1 hantavirus G1 cytoplasmic tail inhibits RIG-I- and TBK-1-directed interferon responses. J. Virol. 809676-9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baffert, F., T. Le, G. Thurston, and D. M. McDonald. 2006. Angiopoietin-1 decreases plasma leakage by reducing number and size of endothelial gaps in venules. Am. J. Physiol. Heart Circ Physiol. 290H107-H118. [DOI] [PubMed] [Google Scholar]
- 3.Baker, E. K., E. C. Tozer, M. Pfaff, S. J. Shattil, J. C. Loftus, and M. H. Ginsberg. 1997. A genetic analysis of integrin function: Glanzmann thrombasthenia in vitro. Proc. Natl. Acad. Sci. USA 941973-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates, D. O., N. J. Hillman, B. Williams, C. R. Neal, and T. M. Pocock. 2002. Regulation of microvascular permeability by vascular endothelial growth factors. J. Anat. 200581-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker, P. M., J. Waltenberger, R. Yachechko, T. Mirzapoiazova, J. S. Sham, C. G. Lee, J. A. Elias, and A. D. Verin. 2005. Neuropilin-1 regulates vascular endothelial growth factor-mediated endothelial permeability. Circ. Res. 961257-1265. [DOI] [PubMed] [Google Scholar]
- 6.Bobik, R., Y. Hong, G. Breier, J. F. Martin, and J. D. Erusalimsky. 1998. Thrombopoietin stimulates VEGF release from c-Mpl-expressing cell lines and hematopoietic progenitors. FEBS Lett. 42310-14. [DOI] [PubMed] [Google Scholar]
- 7.Brinkmann, V. 2007. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol. Ther. 11584-105. [DOI] [PubMed] [Google Scholar]
- 8.Bustamante, E. A., H. Levy, and S. Q. Simpson. 1997. Pleural fluid characteristics in hantavirus pulmonary syndrome. Chest 1121133-1136. [DOI] [PubMed] [Google Scholar]
- 9.Byzova, T. V., C. K. Goldman, N. Pampori, K. A. Thomas, A. Bett, S. J. Shattil, and E. F. Plow. 2000. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol. Cell 6851-860. [PubMed] [Google Scholar]
- 10.Chinoy, M. R., M. M. Graybill, S. A. Miller, C. M. Lang, and G. L. Kauffman. 2002. Angiopoietin-1 and VEGF in vascular development and angiogenesis in hypoplastic lungs. Am. J. Physiol. Lung Cell Mol. Physiol. 283L60-L66. [DOI] [PubMed] [Google Scholar]
- 11.Duchin, J. S., F. T. Koster, C. J. Peters, G. L. Simpson, B. Tempest, S. R. Zaki, T. G. Ksiazek, P. E. Rollin, S. Nichol, E. T. Umland, R. L. Moolenaar, S. E. Reef, K. B. Nolte, M. M. Gallaher, J. C. Butler, R. F. Breiman, et al. 1994. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. N. Engl. J. Med. 330949-955. [DOI] [PubMed] [Google Scholar]
- 12.Dvorak, H. F. 2006. Discovery of vascular permeability factor (VPF). Exp. Cell Res. 312522-526. [DOI] [PubMed] [Google Scholar]
- 13.Dvorak, H. F., T. M. Sioussat, L. F. Brown, B. Berse, J. A. Nagy, A. Sotrel, E. J. Manseau, L. Van de Water, and D. R. Senger. 1991. Distribution of vascular permeability factor (vascular endothelial growth factor) in tumors: concentration in tumor blood vessels. J. Exp. Med. 1741275-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eklund, L., and B. R. Olsen. 2005. Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp. Cell Res. 312630-641. [DOI] [PubMed] [Google Scholar]
- 15.French, D. L., and B. S. Coller. 1997. Hematologically important mutations: Glanzmann thrombasthenia. Blood Cells Mol. Dis. 2339-51. [DOI] [PubMed] [Google Scholar]
- 16.Gamble, J. R., J. Drew, L. Trezise, A. Underwood, M. Parsons, L. Kasminkas, J. Rudge, G. Yancopoulos, and M. A. Vadas. 2000. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ. Res. 87603-607. [DOI] [PubMed] [Google Scholar]
- 17.Gavard, J., and J. S. Gutkind. 2006. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat. Cell Biol. 81223-1234. [DOI] [PubMed] [Google Scholar]
- 18.Gavrilovskaya, I., R. LaMonica, M. E. Fay, B. Hjelle, C. Schmaljohn, R. Shaw, and E. R. Mackow. 1999. New York 1 and Sin Nombre viruses are serotypically distinct viruses associated with hantavirus pulmonary syndrome. J. Clin. Microbiol. 37122-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gavrilovskaya, I. N., E. J. Brown, M. H. Ginsberg, and E. R. Mackow. 1999. Cellular entry of hantaviruses which cause hemorrhagic fever with renal syndrome is mediated by β3 integrins. J. Virol. 733951-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gavrilovskaya, I. N., T. Peresleni, E. Geimonen, and E. R. Mackow. 2002. Pathogenic hantaviruses selectively inhibit beta3 integrin directed endothelial cell migration. Arch. Virol. 1471913-1931. [DOI] [PubMed] [Google Scholar]
- 21.Gavrilovskaya, I. N., M. Shepley, R. Shaw, M. H. Ginsberg, and E. R. Mackow. 1998. Beta3 Integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc. Natl. Acad. Sci. USA 957074-7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harhaj, N. S., A. J. Barber, and D. A. Antonetti. 2002. Platelet-derived growth factor mediates tight junction redistribution and increases permeability in MDCK cells. J. Cell Physiol. 193349-364. [DOI] [PubMed] [Google Scholar]
- 23.Hayasaka, D., K. Maeda, F. A. Ennis, and M. Terajima. 2007. Increased permeability of human endothelial cell line EA.hy926 induced by hantavirus-specific cytotoxic T lymphocytes. Virus Res. 123120-127. [DOI] [PubMed] [Google Scholar]
- 24.Hippenstiel, S., M. Krull, A. Ikemann, W. Risau, M. Clauss, and N. Suttorp. 1998. VEGF induces hyperpermeability by a direct action on endothelial cells. Am. J. Physiol. 274L678-L684. [DOI] [PubMed] [Google Scholar]
- 25.Hodivala-Dilke, K. M., K. P. McHugh, D. A. Tsakiris, H. Rayburn, D. Crowley, M. Ullman-Cullere, F. P. Ross, B. S. Coller, S. Teitelbaum, and R. O. Hynes. 1999. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J. Clin. Investig. 103229-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holmes, K., O. L. Roberts, A. M. Thomas, and M. J. Cross. 2007. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 192003-2012. [DOI] [PubMed] [Google Scholar]
- 27.Hooper, J. W., T. Larsen, D. M. Custer, and C. S. Schmaljohn. 2001. A lethal disease model for hantavirus pulmonary syndrome. Virology 2896-14. [DOI] [PubMed] [Google Scholar]
- 28.Hynes, R. O. 2003. Structural biology: changing partners. Science 300755-756. [DOI] [PubMed] [Google Scholar]
- 29.Hynes, R. O., and K. M. Hodivala-Dilke. 1999. Insights and questions arising from studies of a mouse model of Glanzmann thrombasthenia. Thromb. Haemost. 82481-485. [PubMed] [Google Scholar]
- 30.Igarashi, J., P. A. Erwin, A. P. Dantas, H. Chen, and T. Michel. 2003. VEGF induces S1P1 receptors in endothelial cells: implications for cross-talk between sphingolipid and growth factor receptors. Proc. Natl. Acad. Sci. USA 10010664-10669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jho, D., D. Mehta, G. Ahmmed, X. P. Gao, C. Tiruppathi, M. Broman, and A. B. Malik. 2005. Angiopoietin-1 opposes VEGF-induced increase in endothelial permeability by inhibiting TRPC1-dependent Ca2 influx. Circ. Res. 961282-1290. [DOI] [PubMed] [Google Scholar]
- 32.Joutsi-Korhonen, L., S. Preston, P. A. Smethurst, M. Ijsseldijk, E. Schaffner-Reckinger, K. L. Armour, N. A. Watkins, M. R. Clark, P. G. de Groot, R. W. Farndale, W. H. Ouwehand, and L. M. Williamson. 2004. The effect of recombinant IgG antibodies against the leucine-33 form of the platelet beta3 integrin (HPA-1a) on platelet function. Thromb. Haemost. 91743-754. [DOI] [PubMed] [Google Scholar]
- 33.Kamba, T., B. Y. Tam, H. Hashizume, A. Haskell, B. Sennino, M. R. Mancuso, S. M. Norberg, M. O'Brien, S., R. B. Davis, L. C. Gowen, K. D. Anderson, G. Thurston, S. Joho, M. L. Springer, C. J. Kuo, and D. M. McDonald. 2006. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am. J. Physiol. Heart Circ. Physiol. 290H560-H576. [DOI] [PubMed] [Google Scholar]
- 34.Kanerva, M., J. Mustonen, and A. Vaheri. 1998. Pathogenesis of Puumala and other hantavirus infections. Rev. Med. Virol. 867-86. [DOI] [PubMed] [Google Scholar]
- 35.Khaiboullina, S. F., D. M. Netski, P. Krumpe, and S. C. St Jeor. 2000. Effects of tumor necrosis factor alpha on Sin Nombre virus infection in vitro. J. Virol. 7411966-11971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirito, K., and K. Kaushansky. 2005. Thrombopoietin stimulates vascular endothelial cell growth factor (VEGF) production in hematopoietic stem cells. Cell Cycle 41729-1731. [DOI] [PubMed] [Google Scholar]
- 37.Klingstrom, J., A. Plyusnin, A. Vaheri, and A. Lundkvist. 2002. Wild-type Puumala hantavirus infection induces cytokines, C-reactive protein, creatinine, and nitric oxide in cynomolgus macaques. J. Virol. 76444-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kobayashi, H., and P. C. Lin. 2005. Angiopoietin/Tie2 signaling, tumor angiogenesis and inflammatory diseases. Front. Biosci. 10666-674. [DOI] [PubMed] [Google Scholar]
- 39.Koster, F., K. Foucar, B. Hjelle, A. Scott, Y. Y. Chong, R. Larson, and M. McCabe. 2001. Rapid presumptive diagnosis of hantavirus cardiopulmonary syndrome by peripheral blood smear review. Am. J. Clin. Pathol. 116665-672. [DOI] [PubMed] [Google Scholar]
- 40.Linderholm, M., C. Ahlm, B. Settergren, A. Waage, and A. Tarnvik. 1996. Elevated plasma levels of tumor necrosis factor (TNF)-alpha, soluble TNF receptors, interleukin (IL)-6, and IL-10 in patients with hemorrhagic fever with renal syndrome. J. Infect. Dis. 17338-43. [DOI] [PubMed] [Google Scholar]
- 41.Mammoto, T., S. M. Parikh, A. Mammoto, D. Gallagher, B. Chan, G. Mostoslavsky, D. E. Ingber, and V. P. Sukhatme. 2007. Angiopoietin-1 requires p190 RhoGAP to protect against vascular leakage in vivo. J. Biol. Chem. 28223910-23918. [DOI] [PubMed] [Google Scholar]
- 42.McCarter, S. D., S. H. Mei, P. F. Lai, Q. W. Zhang, C. H. Parker, R. S. Suen, R. D. Hood, Y. D. Zhao, Y. Deng, R. N. Han, D. J. Dumont, and D. J. Stewart. 2007. Cell-based angiopoietin-1 gene therapy for acute lung injury. Am. J. Respir. Crit. Care Med. 1751014-1026. [DOI] [PubMed] [Google Scholar]
- 43.McVerry, B. J., and J. G. Garcia. 2004. Endothelial cell barrier regulation by sphingosine 1-phosphate. J. Cell Biochem. 921075-1085. [DOI] [PubMed] [Google Scholar]
- 44.McVerry, B. J., and J. G. Garcia. 2005. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cell Signal. 17131-139. [DOI] [PubMed] [Google Scholar]
- 45.Medford, A. R., and A. B. Millar. 2006. Vascular endothelial growth factor (VEGF) in acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): paradox or paradigm? Thorax 61621-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mita, M. M., E. K. Rowinsky, L. Forero, S. G. Eckhart, E. Izbicka, G. R. Weiss, M. Beeram, A. C. Mita, J. S. de Bono, A. W. Tolcher, L. A. Hammond, P. Simmons, K. Berg, C. Takimoto, and A. Patnaik. 2007. A phase II, pharmacokinetic, and biologic study of semaxanib and thalidomide in patients with metastatic melanoma. Cancer Chemother. Pharmacol. 59165-174. [DOI] [PubMed] [Google Scholar]
- 47.Moreira, I. S., P. A. Fernandes, and M. J. Ramos. 2007. Vascular endothelial growth factor (VEGF) inhibition: a critical review. Anticancer Agents Med. Chem. 7223-245. [DOI] [PubMed] [Google Scholar]
- 48.Mukhopadhyay, D., H. Zeng, and R. Bhattacharya. 2004. Complexity in the vascular permeability factor/vascular endothelial growth factor (VPF/VEGF)-receptors signaling. Mol. Cell Biochem. 26451-61. [DOI] [PubMed] [Google Scholar]
- 49.Nolte, K. B., R. M. Feddersen, K. Foucar, S. R. Zaki, F. T. Koster, D. Madar, T. L. Merlin, P. J. McFeeley, E. T. Umland, and R. E. Zumwalt. 1995. Hantavirus pulmonary syndrome in the United States: a pathological description of a disease caused by a new agent. Hum. Pathol. 26110-120. [DOI] [PubMed] [Google Scholar]
- 50.Olsson, A. K., A. Dimberg, J. Kreuger, and L. Claesson-Welsh. 2006. VEGF receptor signalling: in control of vascular function. Nat. Rev. Mol. Cell. Biol. 7359-371. [DOI] [PubMed] [Google Scholar]
- 51.Pizurki, L., Z. Zhou, K. Glynos, C. Roussos, and A. Papapetropoulos. 2003. Angiopoietin-1 inhibits endothelial permeability, neutrophil adherence and IL-8 production. Br. J. Pharmacol. 139329-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pocock, T. M., R. R. Foster, and D. O. Bates. 2004. Evidence of a role for TRPC channels in VEGF-mediated increased vascular permeability in vivo. Am. J. Physiol. Heart Circ. Physiol. 286H1015-H1026. [DOI] [PubMed] [Google Scholar]
- 53.Rasche, F. M., B. Uhel, D. H. Kruger, W. Karges, D. Czock, W. Hampl, F. Keller, H. Meisel, and L. von Muller. 2004. Thrombocytopenia and acute renal failure in Puumala hantavirus infections. Emerg. Infect. Dis. 101420-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raymond, T., E. Gorbunova, I. N. Gavrilovskaya, and E. R. Mackow. 2005. Pathogenic hantaviruses bind plexin-semaphorin-integrin domains present at the apex of inactive, bent αvβ3 integrin conformers. Proc. Natl. Acad. Sci. USA 1021163-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reynolds, L. E., L. Wyder, J. C. Lively, D. Taverna, S. D. Robinson, X. Huang, D. Sheppard, R. O. Hynes, and K. M. Hodivala-Dilke. 2002. Enhanced pathological angiogenesis in mice lacking β3 integrin or β3 and β5 integrins. Nat. Med. 827-34. [DOI] [PubMed] [Google Scholar]
- 56.Ridley, A. J., M. A. Schwartz, K. Burridge, R. A. Firtel, M. H. Ginsberg, G. Borisy, J. T. Parsons, and A. R. Horwitz. 2003. Cell migration: integrating signals from front to back. Science 3021704-1709. [DOI] [PubMed] [Google Scholar]
- 57.Robinson, S. D., L. E. Reynolds, L. Wyder, D. J. Hicklin, and K. M. Hodivala-Dilke. 2004. β3-integrin regulates vascular endothelial growth factor-A-dependent permeability. Arterioscler. Thromb. Vasc. Biol. 242108-2114. [DOI] [PubMed] [Google Scholar]
- 58.Rozman, P. 2002. Platelet antigens: the role of human platelet alloantigens (HPA) in blood transfusion and transplantation. Transpl. Immunol. 10165-181. [DOI] [PubMed] [Google Scholar]
- 59.Sanchez, T., T. Estrada-Hernandez, J. H. Paik, M. T. Wu, K. Venkataraman, V. Brinkmann, K. Claffey, and T. Hla. 2003. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J. Biol. Chem. 27847281-47290. [DOI] [PubMed] [Google Scholar]
- 60.Satchell, S. C., K. L. Anderson, and P. W. Mathieson. 2004. Angiopoietin 1 and vascular endothelial growth factor modulate human glomerular endothelial cell barrier properties. J. Am. Soc. Nephrol. 15566-574. [DOI] [PubMed] [Google Scholar]
- 61.Satchell, S. C., S. J. Harper, J. E. Tooke, D. Kerjaschki, M. A. Saleem, and P. W. Mathieson. 2002. Human podocytes express angiopoietin 1, a potential regulator of glomerular vascular endothelial growth factor. J. Am. Soc. Nephrol. 13544-550. [DOI] [PubMed] [Google Scholar]
- 62.Satchell, S. C., and P. W. Mathieson. 2003. Angiopoietins: microvascular modulators with potential roles in glomerular pathophysiology. J. Nephrol. 16168-178. [PubMed] [Google Scholar]
- 63.Schaphorst, K. L., E. Chiang, K. N. Jacobs, A. Zaiman, V. Natarajan, F. Wigley, and J. G. Garcia. 2003. Role of sphingosine-1 phosphate in the enhancement of endothelial barrier integrity by platelet-released products. Am. J. Physiol. Lung Cell Mol. Physiol. 285L258-L267. [DOI] [PubMed] [Google Scholar]
- 64.Schmaljohn, C. 2001. Bunyaviridae and their replication, p. 1581-1602. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott-Raven Publishers, Philadelphia, PA.
- 65.Schmaljohn, C., and B. Hjelle. 1997. Hantaviruses: a global disease problem. Emerg. Infect. Dis. 395-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmid, G., M. Guba, I. Ischenko, A. Papyan, M. Joka, S. Schrepfer, C. J. Bruns, K. W. Jauch, C. Heeschen, and C. Graeb. 2007. The immunosuppressant FTY720 inhibits tumor angiogenesis via the sphingosine 1-phosphate receptor 1. J. Cell Biochem. 101259-270. [DOI] [PubMed] [Google Scholar]
- 67.Seebach, J., H. J. Madler, B. Wojciak-Stothard, and H. J. Schnittler. 2005. Tyrosine phosphorylation and the small GTPase rac cross-talk in regulation of endothelial barrier function. Thromb. Haemost. 94620-629. [DOI] [PubMed] [Google Scholar]
- 68.Senger, D. R., S. R. Ledbetter, K. P. Claffey, A. Papadopoulos-Sergiou, C. A. Peruzzi, and M. Detmar. 1996. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the αvβ3 integrin, osteopontin, and thrombin. Am. J. Pathol. 149293-305. [PMC free article] [PubMed] [Google Scholar]
- 69.Soldi, R., S. Mitola, M. Strasly, P. Defilippi, G. Tarone, and F. Bussolino. 1999. Role of αvβ3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 18882-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Srikiatkhachorn, A., C. Ajariyakhajorn, T. P. Endy, S. Kalayanarooj, D. H. Libraty, S. Green, F. A. Ennis, and A. L. Rothman. 2007. Virus-induced decline in soluble vascular endothelial growth receptor 2 is associated with plasma leakage in dengue hemorrhagic fever. J. Virol. 811592-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sugimori, T., D. L. Griffith, and M. A. Arnaout. 1997. Emerging paradigms of integrin ligand binding and activation. Kidney Int. 511454-1462. [DOI] [PubMed] [Google Scholar]
- 72.Sundstrom, J. B., L. K. McMullan, C. F. Spiropoulou, W. C. Hooper, A. A. Ansari, C. J. Peters, and P. E. Rollin. 2001. Hantavirus infection induces the expression of RANTES and IP-10 without causing increased permeability in human lung microvascular endothelial cells. J. Virol. 756070-6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takagi, J., B. M. Petre, T. Walz, and T. A. Springer. 2002. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 110599-611. [DOI] [PubMed] [Google Scholar]
- 74.Takuwa, Y., N. Takuwa, and N. Sugimoto. 2002. The Edg family G protein-coupled receptors for lysophospholipids: their signaling properties and biological activities. J. Biochem. 131767-771. [DOI] [PubMed] [Google Scholar]
- 75.Tani, M., T. Sano, M. Ito, and Y. Igarashi. 2005. Mechanisms of sphingosine and sphingosine 1-phosphate generation in human platelets. J. Lipid Res. 462458-2467. [DOI] [PubMed] [Google Scholar]
- 76.Terajima, M., D. Hayasaka, K. Maeda, and F. A. Ennis. 2007. Immunopathogenesis of hantavirus pulmonary syndrome and hemorrhagic fever with renal syndrome: do CD8+ T cells trigger capillary leakage in viral hemorrhagic fevers? Immunol. Lett. 113117-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thurston, G., J. S. Rudge, E. Ioffe, H. Zhou, L. Ross, S. D. Croll, N. Glazer, J. Holash, D. M. McDonald, and G. D. Yancopoulos. 2000. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat. Med. 6460-463. [DOI] [PubMed] [Google Scholar]
- 78.Thurston, G., C. Suri, K. Smith, J. McClain, T. N. Sato, G. D. Yancopoulos, and D. M. McDonald. 1999. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 2862511-2514. [DOI] [PubMed] [Google Scholar]
- 79.Van Nieuw Amerongen, G. P., and V. W. Van Hinsbergh. 1999. Determination of the endothelial barrier function in vitro. Methods Mol. Biol. 96183-189. [DOI] [PubMed] [Google Scholar]
- 80.Vapalahti, O., A. Lundkvist, S. K. Kukkonen, Y. Cheng, M. Gilljam, M. Kanerva, T. Manni, M. Pejcoch, J. Niemimaa, A. Kaikusalo, H. Henttonen, A. Vaheri, and A. Plyusnin. 1996. Isolation and characterization of Tula virus, a distinct serotype in the genus Hantavirus, family Bunyaviridae. J. Gen. Virol. 77(Pt. 12)3063-3067. [DOI] [PubMed] [Google Scholar]
- 81.Verheul, H. M., A. S. Jorna, K. Hoekman, H. J. Broxterman, M. F. Gebbink, and H. M. Pinedo. 2000. Vascular endothelial growth factor-stimulated endothelial cells promote adhesion and activation of platelets. Blood 964216-4221. [PubMed] [Google Scholar]
- 82.Vinores, S. A., W. H. Xiao, J. Shen, and P. A. Campochiaro. 2007. TNF-alpha is critical for ischemia-induced leukostasis, but not retinal neovascularization nor VEGF-induced leakage. J. Neuroimmunol. 18273-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Voelkel, N. F., R. W. Vandivier, and R. M. Tuder. 2006. Vascular endothelial growth factor in the lung. Am. J. Physiol. Lung Cell Mol. Physiol. 290L209-L221. [DOI] [PubMed] [Google Scholar]
- 84.Wahl-Jensen, V., J. Chapman, L. Asher, R. Fisher, M. Zimmerman, T. Larsen, and J. W. Hooper. 2007. Temporal analysis of Andes virus and Sin Nombre virus infections of Syrian hamsters. J. Virol. 817449-7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wallez, Y., I. Vilgrain, and P. Huber. 2006. Angiogenesis: the VE-cadherin switch. Trends Cardiovasc. Med. 1655-59. [DOI] [PubMed] [Google Scholar]
- 86.Wang, Y., S. Pampou, K. Fujikawa, and L. Varticovski. 2004. Opposing effect of angiopoietin-1 on VEGF-mediated disruption of endothelial cell-cell interactions requires activation of PKC beta. J. Cell Physiol. 19853-61. [DOI] [PubMed] [Google Scholar]
- 87.Watkins, N. A., P. A. Smethurst, D. Allen, G. A. Smith, and W. H. Ouwehand. 2002. Platelet αIIbβ3 recombinant autoantibodies from the B-cell repertoire of a posttransfusion purpura patient. Br. J. Hematol. 116677-685. [DOI] [PubMed] [Google Scholar]
- 88.Weis, S. M., and D. A. Cheresh. 2005. Pathophysiological consequences of VEGF-induced vascular permeability. Nature 437497-504. [DOI] [PubMed] [Google Scholar]
- 89.Woolf, A. S. 2005. Molecular and genetic analyses of renal capillary development: studying the angiopoietin/Tie axis. Kidney Int. 681968. [Google Scholar]
- 90.Xiao, R., S. Yang, F. Koster, C. Ye, C. Stidley, and B. Hjelle. 2006. Sin Nombre viral RNA load in patients with hantavirus cardiopulmonary syndrome. J. Infect. Dis. 1941403-1409. [DOI] [PubMed] [Google Scholar]
- 91.Xu, M., C. L. Waters, C. Hu, R. B. Wysolmerski, P. A. Vincent, and F. L. Minnear. 2007. Sphingosine 1-phosphate rapidly increases endothelial barrier function independently of VE-cadherin but requires cell spreading and Rho kinase. Am. J. Physiol. Cell Physiol. 2931309-1318. [DOI] [PubMed] [Google Scholar]
- 92.Yamazaki, Y., Y. Nakano, T. Imamura, and T. Morita. 2007. Augmentation of vascular permeability of VEGF is enhanced by KDR-binding proteins. Biochem. Biophys. Res. Commun. 355693-699. [DOI] [PubMed] [Google Scholar]
- 93.Zaki, S., P. Greer, L. Coffield, C. Goldsmith, K. Nolte, K. Foucar, R. Feddersen, R. Zumwalt, G. Miller, P. Rollin, T. Ksiazek, S. Nichol, and C. Peters. 1995. Hantavirus pulmonary syndrome: pathogenesis of an emerging infectious disease. Am. J. Pathol. 146552-579. [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang, J. L., J. L. Wang, N. Gao, Z. T. Chen, Y. P. Tian, and J. An. 2007. Up-regulated expression of β3 integrin induced by dengue virus serotype 2 infection associated with virus entry into human dermal microvascular endothelial cells. Biochem. Biophys. Res. Commun. 356763-768. [DOI] [PubMed] [Google Scholar]