Abstract
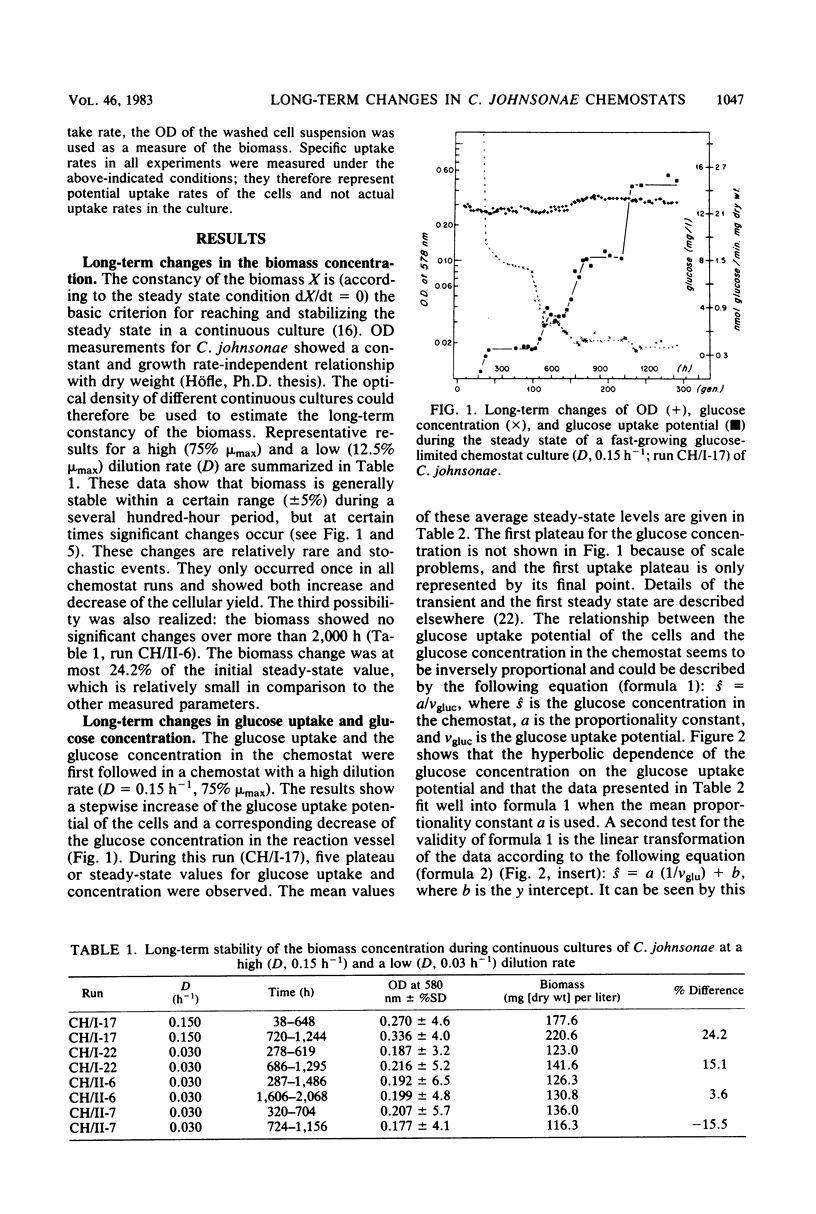
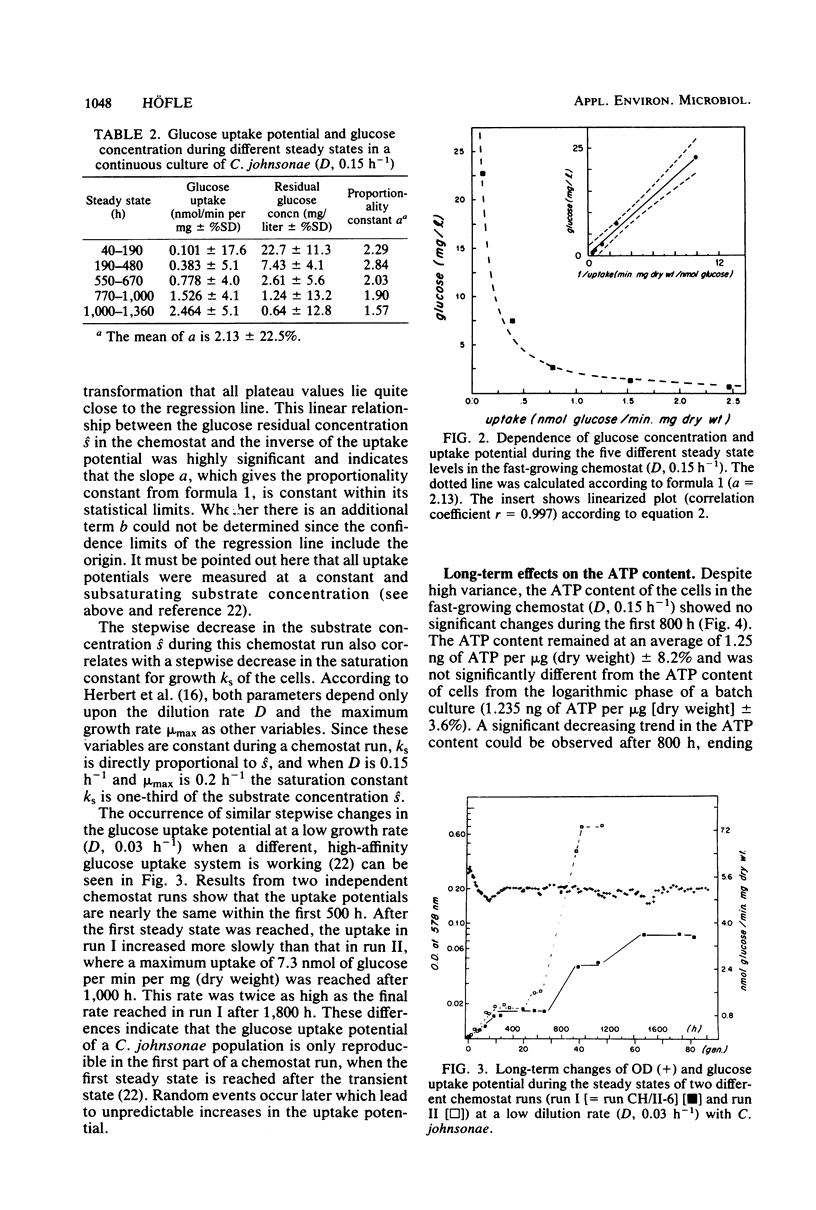
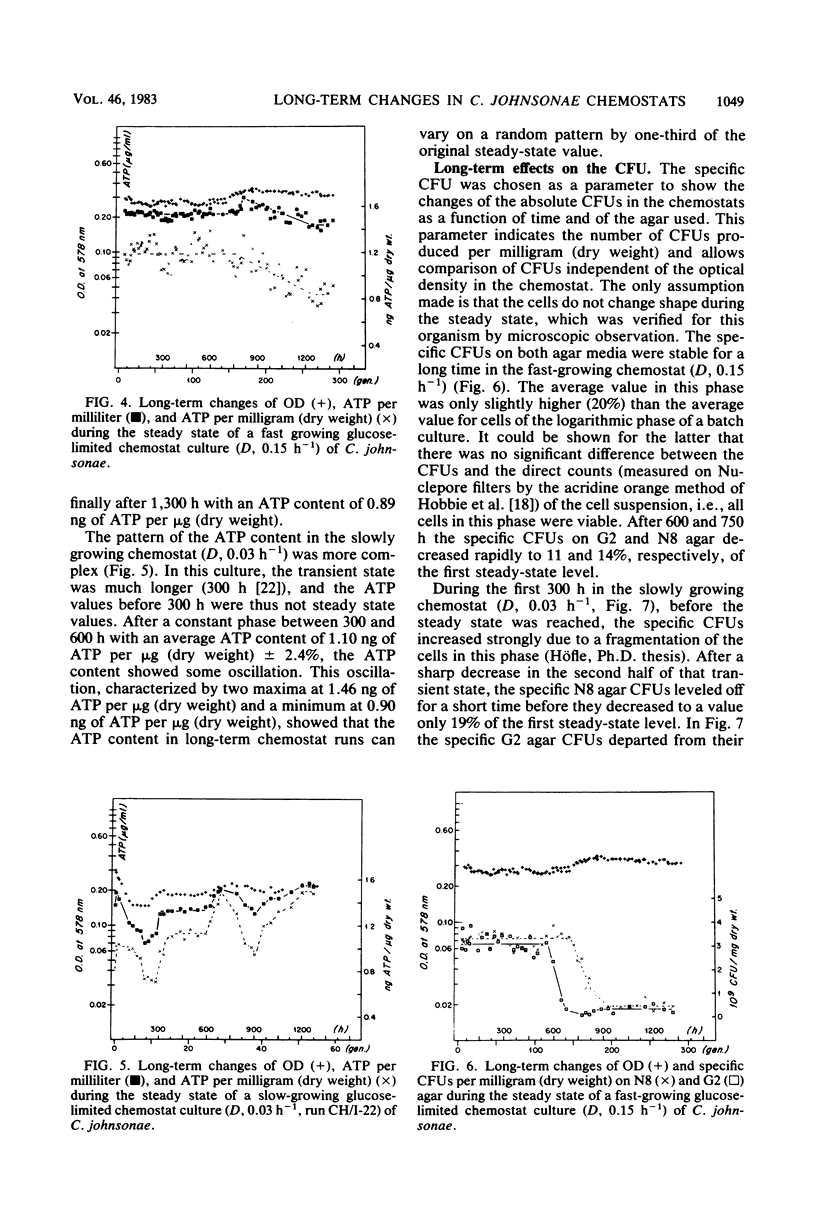
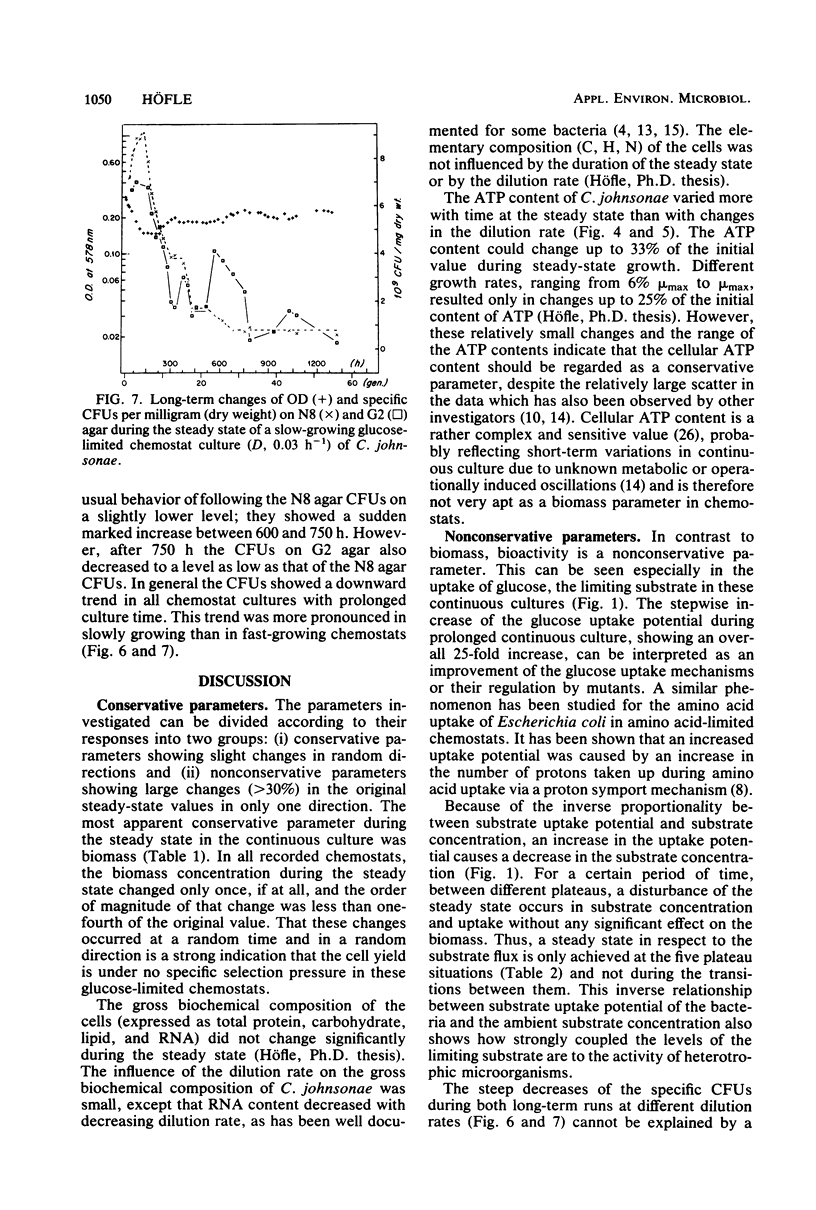
Long-term studies with a gliding, heterotrophic bacterium, Cytophaga johnsonae, were conducted in a glucose-limited chemostat at a high and a low dilution rate. To test the stability of the steady state during long-term experiments the following parameters were monitored: optical density, glucose concentration, glucose uptake potential, ATP content of the cells, and plate counts on two different agar media. Biomass remained relatively constant, although the observed changes could have been possible in both directions. During all steady states, glucose uptake showed a stepwise increase and the glucose concentration showed a corresponding decrease. Glucose uptake potential and glucose concentration in the chemostat were inversely proportional. The ATP content of the cells varied up to 33% during the steady state, but did not show a general trend. After long cultivation in all chemostats, plate counts on both agars dropped to values less than 20% of the original steady-state level. These decreases were due to an inability of the cells to grow on agar plates, not to a lack of vitality of the cells in the chemostat. This study showed that even during shorter chemostat runs, e.g., 1 week, changes in important parameters with the steady state must be expected, especially in the uptake potential and the concentration of the limiting substrate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell C. R., Holder-Franklin M. A., Franklin M. Correlations between predominant heterotrophic bacteria and physicochemical water quality parameters in two canadian rivers. Appl Environ Microbiol. 1982 Feb;43(2):269–283. doi: 10.1128/aem.43.2.269-283.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavari B. Z., Phelps G. Sensitive enzymatic assay for glucose determination in natural waters. Appl Environ Microbiol. 1977 Jun;33(6):1237–1243. doi: 10.1128/aem.33.6.1237-1243.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen P. J. The history, biology, and taxonomy of the Cytophaga group. Can J Microbiol. 1977 Dec;23(12):1599–1653. doi: 10.1139/m77-236. [DOI] [PubMed] [Google Scholar]
- Collins S. H., Jarvis A. W., Lindsay R. J., Hamilton W. A. Proton movements coupled to lactate and alanine transport in Escherichia coli: isolation of mutants with altered stoichiometry in alanine transport. J Bacteriol. 1976 Jun;126(3):1232–1244. doi: 10.1128/jb.126.3.1232-1244.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox E. C., Gibson T. C. Selection for high mutation rates in chemostats. Genetics. 1974 Jun;77(2):169–184. doi: 10.1093/genetics/77.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest W. W. Energies of activation and uncoupled growth in Streptococcus faecalis and Zymomonas mobilis. J Bacteriol. 1967 Nov;94(5):1459–1463. doi: 10.1128/jb.94.5.1459-1463.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güde H. Occurrence of cytophagas in sewage plants. Appl Environ Microbiol. 1980 Apr;39(4):756–763. doi: 10.1128/aem.39.4.756-763.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERBERT D., ELSWORTH R., TELLING R. C. The continuous culture of bacteria; a theoretical and experimental study. J Gen Microbiol. 1956 Jul;14(3):601–622. doi: 10.1099/00221287-14-3-601. [DOI] [PubMed] [Google Scholar]
- HORIUCHI T., TOMIZAWA J. I., NOVICK A. Isolation and properties of bacteria capable of high rates of beta-galactosidase synthesis. Biochim Biophys Acta. 1962 Jan 22;55:152–163. doi: 10.1016/0006-3002(62)90941-1. [DOI] [PubMed] [Google Scholar]
- Harder W., Kuenen J. G. A review. Microbial selection in continuous culture. J Appl Bacteriol. 1977 Aug;43(1):1–24. doi: 10.1111/j.1365-2672.1977.tb00717.x. [DOI] [PubMed] [Google Scholar]
- Harder W., Veldkamp H. A continuous culture study of an obligately psychrophilic Pseudomonas species. Arch Mikrobiol. 1967;59(1):123–130. doi: 10.1007/BF00406323. [DOI] [PubMed] [Google Scholar]
- Herbert D., Kornberg H. L. Glucose transport as rate-limiting step in the growth of Escherichia coli on glucose. Biochem J. 1976 May 15;156(2):477–480. doi: 10.1042/bj1560477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl D. M. Cellular nucleotide measurements and applications in microbial ecology. Microbiol Rev. 1980 Dec;44(4):739–796. doi: 10.1128/mr.44.4.739-796.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L. The adaptive responses of Escherichia coli to a feast and famine existence. Adv Microb Physiol. 1971;6:147–217. doi: 10.1016/s0065-2911(08)60069-7. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Wang C. H. How close to the theoretical diffusion limit do bacterial uptake systems function? Arch Microbiol. 1982 Feb;131(1):36–42. doi: 10.1007/BF00451496. [DOI] [PubMed] [Google Scholar]
- Lundin A., Thore A. Comparison of methods for extraction of bacterial adenine nucleotides determined by firefly assay. Appl Microbiol. 1975 Nov;30(5):713–721. doi: 10.1128/am.30.5.713-721.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK A., SZILARD L. Experiments with the Chemostat on spontaneous mutations of bacteria. Proc Natl Acad Sci U S A. 1950 Dec;36(12):708–719. doi: 10.1073/pnas.36.12.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. ACCELERATED DEATH OF AEROBACTER AEROGENES STARVED IN THE PRESENCE OF GROWTH-LIMITING SUBSTRATES. J Gen Microbiol. 1964 Mar;34:459–473. doi: 10.1099/00221287-34-3-459. [DOI] [PubMed] [Google Scholar]


