Abstract
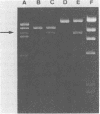
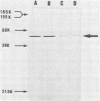
The structural gene for a thermostable α-amylase from Bacillus stearothermophilus was cloned in plasmids pTB90 and pTB53. It was expressed in both B. stearothermophilus and Bacillus subtilis. B. stearothermophilus carrying the recombinant plasmid produced about fivefold more α-amylase (20.9 U/mg of dry cells) than did the wild-type strain of B. stearothermophilus. Some properties of the α-amylases that were purified from the transformants of B. stearothermophilus and B. subtilis were examined. No significant differences were observed among the enzyme properties despite the difference in host cells. It was found that the α-amylase, with a molecular weight of 53,000, retained about 60% of its activity even after treatment at 80°C for 60 min.
Full text
PDF

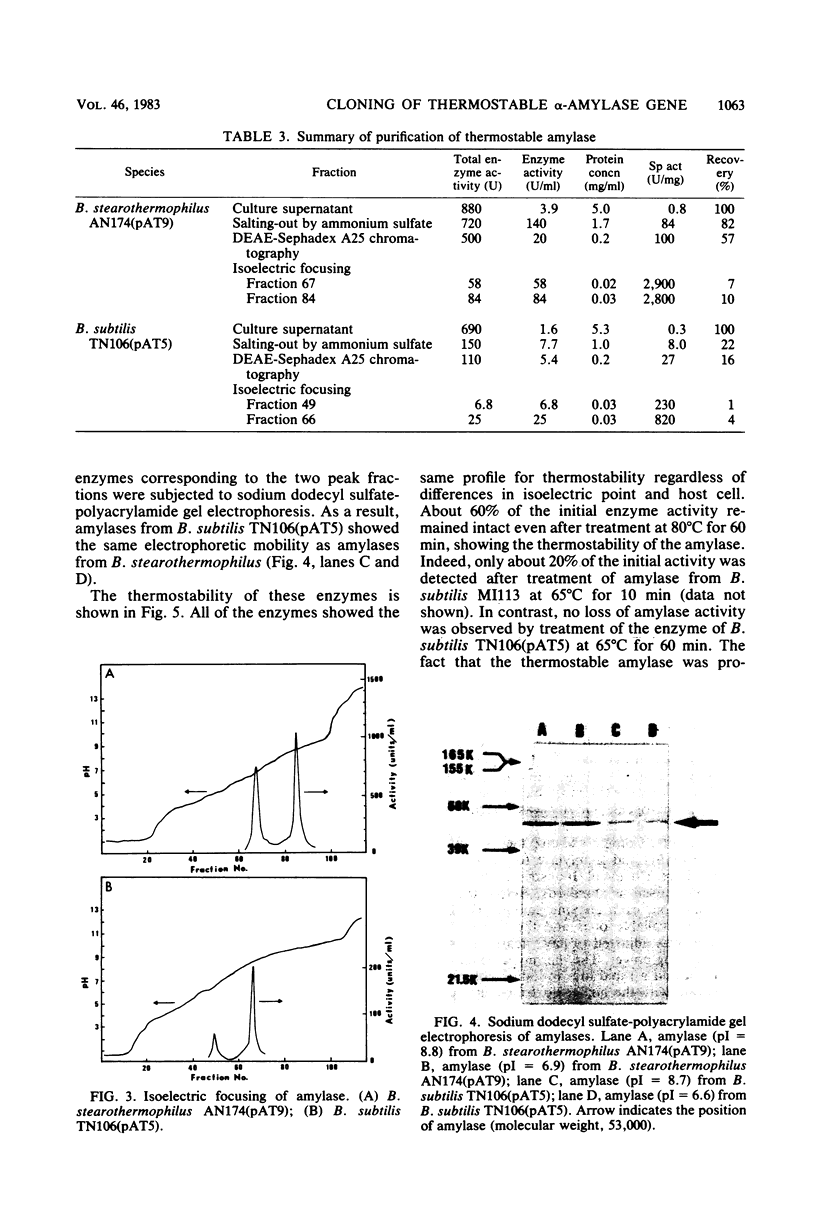
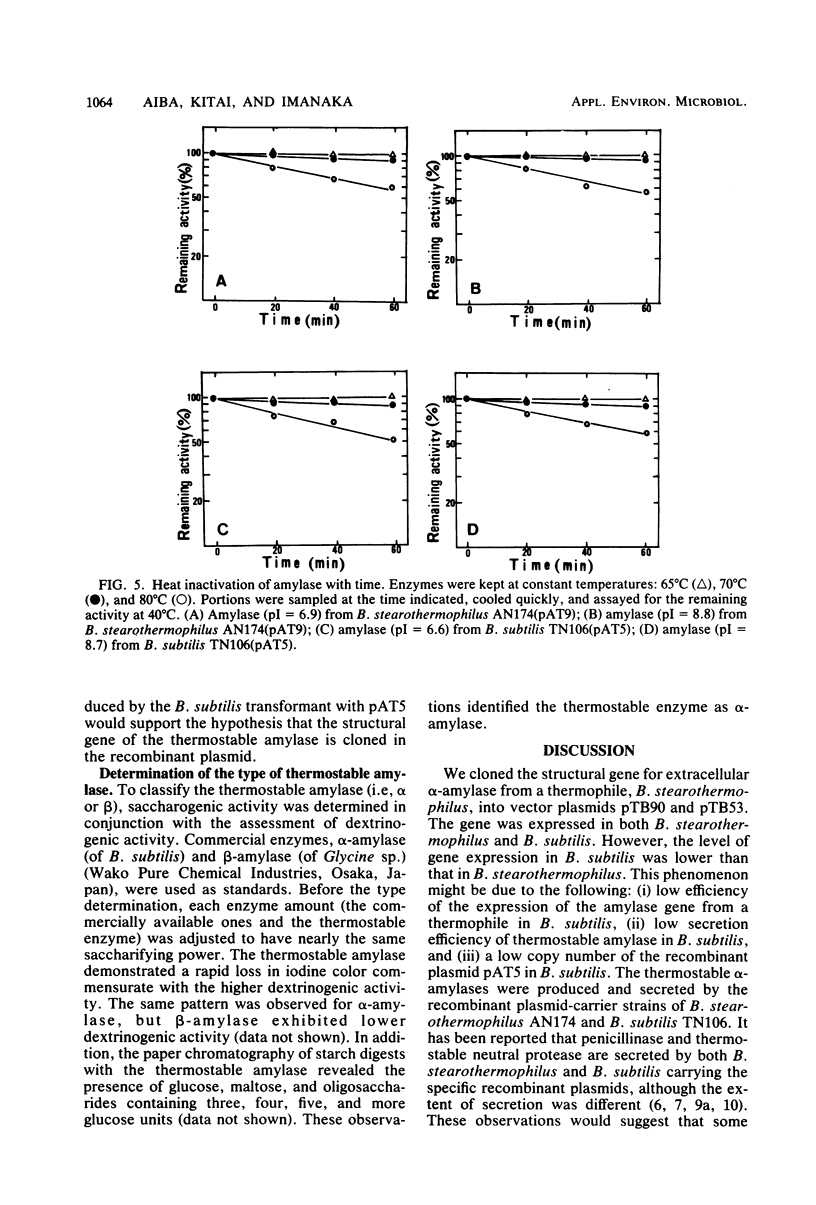

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Cornelis P., Digneffe C., Willemot K. Cloning and expression of a Bacillus coagulans amylase gene in Escherichia coli. Mol Gen Genet. 1982;186(4):507–511. doi: 10.1007/BF00337957. [DOI] [PubMed] [Google Scholar]
- Fujii M., Takagi M., Imanaka T., Aiba S. Molecular cloning of a thermostable neutral protease gene from Bacillus stearothermophilus in a vector plasmid and its expression in Bacillus stearothermophilus and Bacillus subtilis. J Bacteriol. 1983 May;154(2):831–837. doi: 10.1128/jb.154.2.831-837.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanaka T., Fujii M., Aiba S. Isolation and characterization of antibiotic resistance plasmids from thermophilic bacilli and construction of deletion plasmids. J Bacteriol. 1981 Jun;146(3):1091–1097. doi: 10.1128/jb.146.3.1091-1097.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanaka T., Fujii M., Aramori I., Aiba S. Transformation of Bacillus stearothermophilus with plasmid DNA and characterization of shuttle vector plasmids between Bacillus stearothermophilus and Bacillus subtilis. J Bacteriol. 1982 Mar;149(3):824–830. doi: 10.1128/jb.149.3.824-830.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanaka T., Oshihara W., Himeno T., Aiba S. Comparative studies on extracellular penicillinases of the same structural gene, penP, expressed in Bacillus licheniformis and Bacillus subtilis. J Gen Microbiol. 1983 Aug;129(8):2621–2628. doi: 10.1099/00221287-129-8-2621. [DOI] [PubMed] [Google Scholar]
- Imanaka T., Tanaka T., Tsunekawa H., Aiba S. Cloning of the genes for penicillinase, penP and penI, of Bacillus licheniformis in some vector plasmids and their expression in Escherichia coli, Bacillus subtilis, and Bacillus licheniformis. J Bacteriol. 1981 Sep;147(3):776–786. doi: 10.1128/jb.147.3.776-786.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANNING G. B., CAMPBELL L. L. Thermostable alpha-amylase of Bacillus stearothermophilus. I. Crystallization and some general properties. J Biol Chem. 1961 Nov;236:2952–2957. [PubMed] [Google Scholar]
- Ogasahara K., Imanishi A., Isemura T. Studies on thermophilic alpha-amylase from Bacillus stearothermophilus. I. Some general and physico-chemical properties of thermophilic alpha-amylase. J Biochem. 1970 Jan;67(1):65–75. doi: 10.1093/oxfordjournals.jbchem.a129235. [DOI] [PubMed] [Google Scholar]
- Palva I. Molecular cloning of alpha-amylase gene from Bacillus amyloliquefaciens and its expression in B. subtilis. Gene. 1982 Jul-Aug;19(1):81–87. doi: 10.1016/0378-1119(82)90191-3. [DOI] [PubMed] [Google Scholar]
- Pfueller S. L., Elliott W. H. The extracellular alpha-amylase of bacillus stearothermophilus. J Biol Chem. 1969 Jan 10;244(1):48–54. [PubMed] [Google Scholar]
- Saito N., Yamamoto K. Regulatory factors affecting alpha-amylase production in bacillus licheniformis. J Bacteriol. 1975 Mar;121(3):848–856. doi: 10.1128/jb.121.3.848-856.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]