Abstract
Intracranial infection of Theiler's murine encephalomyelitis virus (TMEV) induces demyelination and a neurological disease in susceptible SJL/J (SJL) mice that resembles multiple sclerosis. While the virus is cleared from the central nervous system (CNS) of resistant C57BL/6 (B6) mice, it persists in SJL mice. To investigate the role of viral persistence and its accompanying immune responses in the development of demyelinating disease, transgenic mice expressing the P1 region of the TMEV genome (P1-Tg) were employed. Interestingly, P1-Tg mice with the B6 background showed severe reductions in both CD4+ and CD8+ T-cell responses to capsid epitopes, while P1-Tg mice with the SJL background displayed transient reductions following viral infection. Reduced antiviral immune responses in P1-Tg mice led to >100- to 1,000-fold increases in viral persistence at 120 days postinfection in the CNS of mice with both backgrounds. Despite the increased CNS TMEV levels in these P1-Tg mice, B6 P1-Tg mice developed neither neuropathological symptoms nor demyelinating lesions, and SJL P1-Tg mice developed significantly less severe TMEV-induced demyelinating disease. These results strongly suggest that viral persistence alone is not sufficient to induce disease and that the level of T-cell immunity to viral capsid epitopes is critical for the development of demyelinating disease in SJL mice.
Theiler's murine encephalomyelitis virus (TMEV) is a member of the Cardiovirus genus within the Picornaviridae family. TMEV is a common enteric pathogen among wild mice and rarely causes a neurological disease. However, intracerebral (i.c.) infection in susceptible mice, e.g., SJL/J (SJL) mice, reproducibly induces a chronic immune-mediated demyelinating disease, providing an excellent infectious model for multiple sclerosis (10, 25). The precise mechanism of TMEV-induced demyelinating disease (TMEV-IDD) is unknown. However, virus-specific T cells, various proinflammatory chemokines (e.g., monocyte chemoattractant protein 1 and interferon-inducible protein 10), and cytokines (e.g., gamma interferon [IFN-γ] and tumor necrosis factor alpha) in the central nervous system (CNS) are believed to play a critical role (reviewed in references 26 and 44) in this disease process. In addition, the presence of persistent viral infection may lead to demyelinating disease by directly causing host cell lysis (51), which releases sequestrated CNS autoantigens, resulting in the activation of autoreactive T cells (39). The persistence of viral antigens also is believed to perpetuate a massive inflammatory milieu in the CNS by continuously activating virus-specific T cells (25, 33). Thus, it is conceivable that viral persistence is essential for the development of TMEV-IDD. This possibility is consistent with the observation that spontaneously occurring variant viruses with relatively low pathogenicity display reduced levels of viral persistence in the CNS (27), implying that viral persistence is a critical susceptibility factor. Furthermore, genetic studies show that viral persistence levels in the CNS correlate with demyelinating disease levels (7, 34).
In general, virus-specific CD8+ T cells play a pivotal role in limiting viral replication. In resistant C57BL/6 (B6) mice, Db-restricted VP2121-130-specific CD8+ T cells seem to be crucial in viral resolution, as the expression of the Db transgene converts susceptible FVB mice to resistant mice (3). Moreover, when FVB/Db-transgenic (Tg) mice were tolerized by a soluble VP2121-130 peptide infusion, their susceptibility was reinstated; these mice exhibited extensive demyelination and high viral loads (38). Like the CD8+ T cells, vigorous antiviral CD4+ T-cell responses in the early stages of viral infection also are important in acquiring resistance to TMEV-IDD (40). Furthermore, virus-specific antibody responses are required to confer resistance to TMEV infection, especially in the absence of CD8+ T cells (22). These data suggest that various virus-specific immune responses contribute to viral resolution and/or resistance to TMEV-IDD in B6 mice.
The role of immune responses in persistent viral infection in susceptible SJL mice is less clear. CD8+ T cells may offer protection, as β2-microglobin-deficient SJL mice exhibit higher viral loads and exacerbated disease symptoms (4). In addition, a recent study has indicated that the level of virus-specific CD8+ T cells in the CNS of susceptible SJL mice is significantly lower than that of resistant B6 mice in the early stages of viral infection, again strongly suggesting that CD8+ T cells play a protective role (36). Although CD4+ T-cell-mediated immune responses are considered pathogenic (16, 57), virus-specific CD4+ T cells also may provide protection by contributing to viral clearance and limiting viral replication during the early stages of viral infection (6, 9). Virus-specific antibody responses also may contribute to protection, although again the protection is restricted during the early phase of infection (22, 23, 54). Taking these findings together, virus-specific humoral and cellular immune responses seem to contribute to controlling viral loads in susceptible SJL mice, depending upon the stage of viral infection.
Despite numerous studies, the relationship between viral persistence and the pathogenesis of demyelinating disease remains unclear. The removal of either B or T cells from resistant mice results in elevated viral persistence but increased demyelination with/without marginal clinical symptoms or encephalitis, a different neurological disease often without demyelination (6, 22, 41, 42, 45, 47). These results imply that demyelination and/or the development of demyelinating disease is tightly associated with viral persistence. However, early studies also have suggested that immune components are necessary for the development of demyelinating disease, as susceptible mice compromised in their CD4+ T-cell compartment, but not their CD8+ T-cell compartment, failed to develop demyelinating disease (4, 5, 13, 32, 50). Furthermore, viral persistence levels do not necessarily correlate with disease susceptibility among genetically dissimilar mice (8). Therefore, identifying the role of viral persistence in the development of demyelinating disease remains elusive; the question of whether or not viral persistence is necessary and sufficient to develop TMEV-IDD still is unanswered.
To investigate the role of viral persistence and virus-specific immune responses in the pathogenesis of TMEV-IDD, Tg mice expressing the P1 region of the TMEV genome (P1-Tg), which carry viral leader and structural protein genes, were used in this study. P1-Tg mice, expressing high levels of the P1 transgene in the brain and liver, showed significantly reduced levels of virus-specific T-cell and antibody responses, which led to higher viral loads in the CNS compared to those of their littermates. However, immune unresponsiveness to viral capsid epitopes by self tolerance was incomplete in P1-Tg mice with the susceptible SJL background compared to that of mice with the resistant B6 background. Surprisingly, B6 P1-Tg mice maintained their resistance to TMEV-IDD, developing neither demyelination nor symptomatic diseases, despite their significantly compromised antiviral immune responses that lead to high levels of prolonged viral persistence in the CNS comparable to that of susceptible SJL mice. Moreover, SJL P1-Tg mice, displaying elevated viral loads, developed significantly reduced levels of neurological disease. Taken together, these results clearly demonstrate that viral persistence alone is not sufficient to induce TMEV-IDD and that viral loads are not associated with demyelinating disease levels. The level of antiviral immune response to the capsid proteins is rather critical for the pathogenesis of demyelinating disease in susceptible SJL mice.
MATERIALS AND METHODS
Animals.
The P1 region of the TMEV pSBW genome (GenBank accession number DQ401688) was cloned into the EcoRV-NotI site of pcDNA3.1. The linearized P1 region of the TMEV genome after the human cytomegalovirus (hCMV) promoter, voiding the bacterial replication-related gene segment, was injected into fertilized eggs of (B6 × SJL)F1 mice by the Northwestern University Tg facility. P1-expressing (B6 × SJL)F1 founders were backcrossed at least 10 generations to B6 and SJL mice from Jackson Laboratories. Parental and Tg mice were housed and bred in the animal care facility of Northwestern University. All experiments were performed with 6- to 10-week-old female mice under the protocols approved by the institutional Animal Care and Use Committee.
Synthetic peptides and antibodies.
All peptides were purchased from GeneMed Synthesis Inc. and were used as previously described (21). All antibodies used were purchased from BD Biosciences (San Diego, CA).
Virus preparation and infection.
The BeAn strain of TMEV used in this study was generated and propagated (and the titers were determined) in BHK-21 cells grown in Dulbecco's modified Eagle's medium supplemented with 7.5% donor calf serum. For i.c. infection, 30 μl of virus solution (0.2 × 106 to 6 × 106 PFU) was injected into the right cerebral hemisphere of 6- to 8-week-old mice anesthetized with isoflurane. Clinical symptoms of disease were assessed weekly on the following grading scale: grade 0, no clinical signs; grade 1, mild waddling gait or flaccid tail; grade 2, severe waddling gait; grade 3, moderate hind limb paresis; and grade 4, severe hind limb paralysis.
Reverse transcriptase PCR (RT-PCR).
Total cellular RNA from various tissues of P1-Tg mice, including the brain, spinal cord, spleen, thymus, liver, and kidney, was isolated by using Trizol reagents (Invitrogen). First-strand cDNA was synthesized from 1 μg of total RNA by utilizing SuperScript III first-strand synthesis supermix (Invitrogen, CA) at 55°C. The relative concentrations of cDNA were equalized among the groups based upon the level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) amplification (35 cycles) by PCR. The following primers for control GAPDH and the P1 transgene were purchased from Integrated DNA Technologies: GAPDH, 5′-AACTTTGGCATTGTGGAAGG-3′ and 5′-ACACATTGGGGGTAGGAACA-3′, and the P1 transgene, 5′-CCGGAATTCGGAGTTGACAATGCTGAGAAAGG-3′ and 5′-TTGCGGCCGCCTCGAGTTCAAGAATGGGGACTG-3′.
Plaque assay.
Virus titers in the infected CNS tissues were enumerated by a standard plaque assay on BHK-21 monolayers (48). After methanol fixation, 0.1% crystal violet was used to visualize plaques on the monolayer.
Isolation of CNS-infiltrating MNCs.
Mice were perfused with sterile Hanks' balanced salt solution (HBSS), and excised brains and spinal cords were homogenized. CNS-infiltrating mononuclear cells (MNCs) were then enriched in the bottom one-third fraction of a continuous 100% Percoll gradient (Pharmacia, Piscataway, NJ) after centrifugation at 27,000 × g for 30 min as previously described (14).
Intracellular staining of cytokine production.
Freshly isolated CNS-infiltrating MNCs were cultured for 6 h in 96-well round-bottom plates in the presence of relevant or control peptides as previously described (35). Allophycocyanin-conjugated anti-CD8 (clone Ly2) or anti-CD4 (clone L3T4) antibody and phycoerythrin-labeled rat monoclonal anti-IFN-γ (XMG1.2) antibody was used for intracellular cytokine staining. Cells were analyzed on a Becton Dickinson FACSCalibur or FACS Sort flow cytometer. Live cells were gated based on light scatter properties.
Cytokine and antibody ELISA.
Mouse IFN-γ and interleukin-13 (IL-13) enzyme-linked immunosorbent assay (ELISA) kits were purchased from BD Biosciences and R&D Systems, Inc. (Minneapolis, MN), respectively. Cytokine levels in the culture supernatants of lymph node and spleen cells were assessed according to the manufacturer's manual. Briefly, diluted samples were incubated for 2 h with plate-bound capture antibody. Cytokine expression levels were visualized by horseradish peroxidase-conjugated detection antibody in the presence of TMB1 substrate (BioFX Laboratories, Owings Mills, MD). The absorbance at 450 nm was measured. The anti-TMEV antibody response was determined with ELISA using plates coated with UV-inactivated TMEV (UV-TMEV), starting with 1/100-diluted serum samples from infected animals as described elsewhere (20).
T-cell proliferation assay.
T-cell proliferation levels were determined using splenocytes from virus-infected mice. Single-cell suspensions of splenocytes (5 × 105/well) in RPMI medium supplemented with 5 × 10−5 M 2-mercaptoethanol and 0.5% normal syngeneic mouse serum were cultured for 72 h in the presence of 1 or 10 μM peptides or 12.5 μg/ml UV-TMEV. For some experiments, lymph node and spleen cells from mice at 9 to 10 days after immunization with UV-TMEV (30 μg/mouse) in complete Freund's adjuvant were cultured for 3 days in the presence of peptides. Cultures were pulsed with 1 μCi [3H]thymidine for 18 h, and then [3H]thymidine incorporation was determined with a liquid scintillation counter. Data are expressed as the mean counts per minute (cpm) ± the standard errors of the means and/or the stimulation index (SI) ± standard errors of the means of the results from triplicate cultures (the SI is the ratio of peptide stimulation to the medium control).
Statistical analysis.
Data are shown either as the means ± standard deviations (SD) of two to three independent experiments or as one representative example from at least three independent experiments. The significance of differences in the mean values was determined by the Student's t test. P < 0.05 was considered statistically significant.
RESULTS
The P1 transgene is expressed at high levels in the brain and liver of P1-Tg mice with either B6 or SJL background.
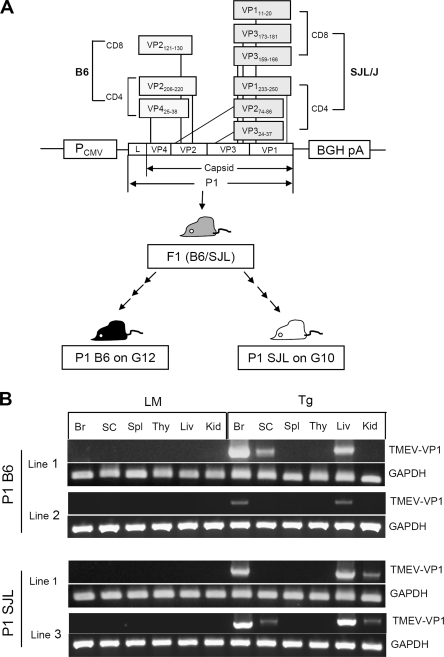
Most viral epitopes recognized by CNS-infiltrating T cells from virus-infected B6 and SJL mice are encoded by the P1 region of the TMEV genome, which contains the sequences for the leader and structural proteins (Fig. 1A). In order to investigate the potential role of TMEV P1-specific immune responses in the development of TMEV-IDD, the induction of unresponsiveness to the P1 region was attempted by the Tg expression of the region. Tg (B6 × SJL)F1 founders, expressing the P1 region of the TMEV genome driven by the hCMV promoter, were bred to either the SJL or B6 background at least 10 generations before use (Fig. 1A). To examine whether the P1 transgene is expressed in these Tg mice, Tg RNA expression levels in various tissues were assessed by RT-PCR (Fig. 1B). cDNA was prepared from the following CNS and peripheral tissues: brain, spinal cord, spleen, thymus, liver, and kidney. The possibility of potential contamination by Tg DNA was excluded by DNase I treatment; the lack of contaminating Tg DNA was further confirmed by PCR with a set of primers specific for the upstream region of the transcription start site and the 3′ end of the leader sequence (data not shown). The VP1 sequence, which is located at the 3′ end of the P1 region, was amplified from cDNA by PCR to analyze the expression of the full-length P1 transgene. Consistently with previous reports regarding proteins expressed under the CMV promoter (28, 52), the P1 transgene was expressed at high levels in the brains and livers of line 1 of both B6 P1-Tg and SJL P1-Tg mice (Fig. 1B). Low levels of transgene expression also were detected in the spinal cord and kidney. B6 P1-Tg line 2 expressed low to negligible levels of the transgene, and SJL P1-Tg line 3 displayed levels of transgene expression comparable to those of line 1. All of these Tg lines, regardless of their P1 transgene expression levels, showed similar reductions in their T-cell responses (data not shown). As P1-Tg line 1 mice with B6 and SJL backgrounds were backcrossed from the same Tg founder, these line 1-derived Tg mice were used for the subsequent experiments.
FIG. 1.
High levels of P1 transgene expression in the brain and liver of B6 and SJL mice. (A) Schematic diagram of the transgene construct. This diagram also shows the P1 epitopes recognized by CNS-infiltrating T cells of TMEV-infected B6 and SJL mice (11, 15, 19, 21, 35, 55, 56). Two minor CD8 epitopes for B6 mice (VP2165-173 and VP3110-120) are not included, as the magnitude of their immune responses are low to negligible. BGH pA, bovine growth hormone polyadenylation signal. (B) The expression of the P1 transgene in various organs of transgenic mice on either B6 or SJL backgrounds. The expression of the P1 transgene was examined by RT-PCR of the brain (Br), spinal cord (SC), spleen (Spl), thymus (Thy), liver (Liv), and kidney (Kid). Transgene-positive mice were used without distinguishing their homozygosity or heterozygosity. Transgene-negative littermates resulting from the backcrossing were used as the littermate (LM) control mice to match the same degree of background genes. Amplification levels of the GAPDH gene are shown for the comparison of cDNA levels in the samples. A representative RT-PCR of two different Tg lines is shown.
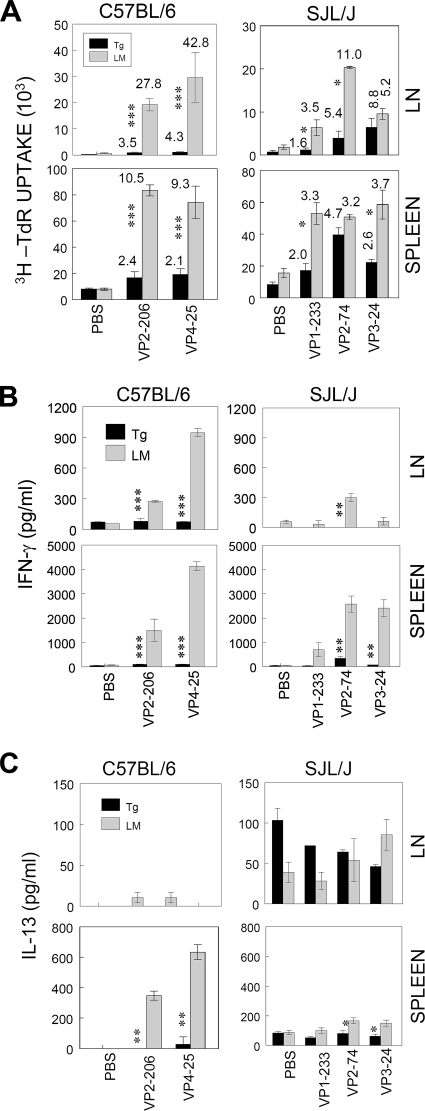
SJL P1-Tg mice immunized with UV-TMEV exhibit lower levels of CD4+ T-cell tolerance to capsid epitopes than B6 P1-Tg mice.
To examine the tolerance levels of T-cell responses to neoself (newself) antigens of viral capsid epitopes, P1-Tg mice and their littermates were immunized with UV-TMEV (Fig. 2). At 9 days postimmunization (pi), lymph node (LN) and spleen cells were stimulated with capsid peptides recognized by CD4+ T cells. B6 P1-Tg mice displayed severe unresponsiveness (P < 0.001) in their proliferative responses to capsid epitopes (VP2206-220 and VP425-38) compared to those from their littermates (Fig. 2A). Overall, T-cell proliferation levels for the epitopes by splenic and LN CD4+ T cells from B6 P1-Tg mice were only 23 and 12% of the responses by their littermates, indicating that CD4+ T-cell responses to P1 neoself antigens were effectively tolerized. In contrast, CD4+ T-cell responses to capsid epitopes (VP1233-250, VP274-86, and VP324-37) in SJL P1-Tg mice were as much as 64 to 75% of the responses by their littermates. These results indicate that the Tg expression of the TMEV P1 region induces the weak tolerance of proliferative responses to capsid-specific CD4+ T-cell epitopes in SJL P1-Tg mice, in contrast to the strong tolerance seen in B6 P1-Tg mice.
FIG. 2.
Inefficient censoring of autoreactive T cells by SJL P1-Tg mice. B6 or SJL P1-Tg mice and their respective littermates (LM) were immunized with UV-TMEV (30 μg) in complete Freund's adjuvant. At 9 to 10 days pi, cells from draining LN and spleens were stimulated with the indicated peptides. (A) The proliferative activity of epitope-specific CD4+ and CD8+ T cells was measured based on [3H]thymidine incorporation levels. The SI, shown at the top of each histogram, was calculated by dividing the cpm of the peptide-stimulated group by that of the phosphate-buffered saline (PBS)-stimulated group. (B and C) IFN-γ and IL-13 levels in the supernatants of the cultures were assessed with ELISA. Values given are the means (±SD) of the results from triplicate wells. *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
To test the possibility that cytokine production levels may be more severely affected than T-cell proliferation levels in these mice, IFN-γ and IL-13 levels produced in the culture supernatant of LN and splenic T cells following stimulation with viral epitopes were assessed by ELISA. LN and spleen cells from B6 P1-Tg mice failed to produce IFN-γ (P < 0.005) and IL-13 (P < 0.01), while their littermates produced high levels of these cytokines (Fig. 2B). Therefore, both proliferative and cytokine responses of capsid-specific CD4+ T cells were greatly reduced in B6 P1-Tg mice. In SJL P1-Tg mice, IFN-γ (P = 0.01) levels also were significantly lower than those of their littermates. However, only the IL-13 levels induced by splenic T cells of P1-Tg mice were marginally lower (P < 0.047) than those of their littermates. IL-13 production by LN cells of SJL Tg mice was not consistent. LN cells from TMEV-immunized SJL Tg mice produce high levels of IL-13 even without any stimulation, unlike that of B6 Tg mice. Therefore, cytokine production by capsid-specific CD4+ T cells appears to be compromised in both B6 and SJL P1-Tg mice, whereas proliferative activity is less affected. However, it is interesting that the CD4+ T-cell tolerance levels were much weaker in SJL P1-Tg mice than in B6 P1-Tg mice.
Initial peripheral CD4+ T-cell response to capsid epitopes is transiently reduced in TMEV-infected P1-Tg mice.
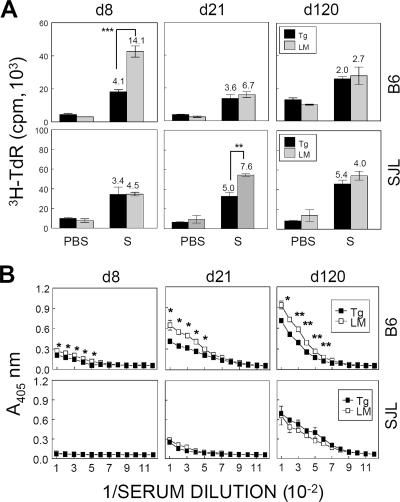
To examine whether the tolerance to viral epitope-specific CD4+ T-cell responses in the periphery also is detected following infection with live virus, spleen cells from TMEV-infected mice after 8, 21, and 120 days pi were stimulated in vitro for 3 days in the presence of capsid epitopes (Fig. 3A). At 8 days pi, splenic T-cell-proliferative response levels of B6 P1-Tg mice were significantly lower (P = 0.0001) than those of their littermates. However, no significant differences in the proliferative responses between the two groups were detected thereafter (Fig. 3A). Interestingly, capsid-specific CD4+ T-cell responses in SJL P1-Tg mice were reduced only at 21 days pi (P < 0.01), while no significant differences were detected at 8 and 120 days pi. Taking these results together, the tolerance of CD4+ T-cell responses to capsid epitopes in the periphery of P1-Tg mice with either the B6 or SJL background is transient following i.c. infection with live TMEV.
FIG. 3.
Levels of peripheral immune responses to viral epitopes in TMEV-infected P1-Tg mice. (A) Splenocytes from mice at 8, 21, and 120 days pi (d8, d21, and d120, respectively) were stimulated with CD4 epitopes. A mixture (S mix) of VP2206-220 and VP425-38 was used for CD4+ T cells from B6 P1-Tg mice and their littermates (top), while a mixture (S mix) of VP1233-250, VP274-86, and VP324-37 was used to stimulate CD4+ T cells from SJL P1-Tg mice and their littermates (bottom). The SI is indicated at the top of each histogram. The values in the figure represent the mean cpm of triplicate cultures (±SD) of a representative experiment. Statistical significance was tested by the Student's t test. *, P < 0.05; **, P < 0.01; and ***, P < 0.001. Three independent experiments were conducted to verify the results, and these are very reproducible. The presented data are from a single experiment because of their variability in the total proliferation levels from one experiment to another. (B) Pooled sera taken from 3 to 10 mice infected with 6 × 106 PFU/mouse were assessed at 8, 21, and 120 days pi for the induction of virus-specific IgG responses. Sera were serially twofold diluted, starting from a 1/100 dilution, and analyzed for TMEV-specific IgG levels. Values given are the means (±SD) of the results from duplicate wells. PBS, phosphate-buffered saline.
Antiviral antibody response is decreased in TMEV-infected B6 P1-Tg mice but not in SJL P1-Tg mice.
To investigate whether the antiviral antibody response also was affected in P1-Tg mice infected with TMEV, antiviral antibody levels in pooled sera from 3 to 10 mice in each group were determined by ELISA at 8, 21, and 120 days pi (Fig. 3B). Interestingly, total virus-specific IgG responses were significantly reduced in B6 P1-Tg mice throughout the course of viral infection compared to those of their littermates, whereas no such difference was apparent between SJL P1-Tg mice and their littermates. The reduction of immunoglobulin G (IgG) responses in B6 P1-Tg mice was not IgG subclass specific, as the levels of both virus-specific IgG1 and IgG2a (or IgG2c) antibodies (37) were similarly reduced (data not shown). These results strongly suggest that the tolerance of antibody responses to viral determinants is significant in B6 P1-Tg mice but not in SJL P1-Tg mice. Therefore, it appears that the tolerance levels of antibody responses to capsid epitopes in P1-Tg mice are different depending upon their genetic backgrounds of resistance and susceptibility.
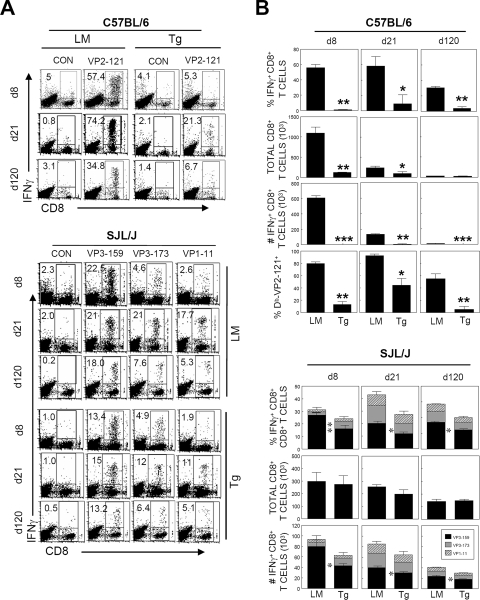
Tolerance of IFN-γ-producing P1-specific CD4+ T-cell responses in the CNS is better maintained in B6 P1-Tg mice than in SJL P1-Tg mice.
To test the possibility that P1-Tg mice are differentially unresponsive to P1-encoded antigens in the CNS of virus-infected mice depending upon their genetic backgrounds, the levels of IFN-γ-producing CNS-infiltrating cells from infected P1-Tg mice with B6 and SJL backgrounds were assessed after stimulation with their respective capsid peptides (Fig. 4). The proportion of virus-specific CD4+ T cells in the CNS of B6 P1-Tg mice was approximately twofold lower than that of their littermates throughout the 120-day course of viral infection, whereas that in SJL P1-Tg mice was lower only during the early (8 days pi) stage of viral infection (Fig. 4A). However, the total number (Fig. 4B) of IFN-γ-producing CD4+ T cells in the CNS of B6 P1-Tg mice was significantly reduced compared to that of their littermates only during the early stage of viral infection (8 days pi) due to the increased infiltration levels at the late stage of viral infection. In SJL P1-Tg mice, differences in the number of P1-specific CD4+ T cells in the CNS also were significant only during early (8 days pi) viral infection (Fig. 4B). Taking these results together, P1-Tg mice with both B6 and SJL backgrounds are able to mount tolerance in P1-specific CD4+ T-cell responses of the CNS during the early stage of TMEV infection, but mice with the B6 background maintain tolerance relatively longer than mice with the SJL background.
FIG. 4.
IFN-γ-producing CD4+ T-cell levels specific to viral epitopes in the CNS of P1-Tg mice. (A) CNS-infiltrating MNCs isolated from TMEV-infected mice were restimulated with the indicated mixture of CD4+ T-cell-specific epitopes (S mix for cells from B6 mice, VP2206-220 and VP425-38; S mix for cells from SJL mice, VP1233-250, VP274-86, and VP324-37). CON represents control cultures similarly incubated with PBS. After a 6-h stimulation, cells were stained for CD4 and intracellular IFN-γ. The percentage of CD4+ and IFN-γ+ cells is shown at the upper left corner of each plot. Data are representative of two to three independent experiments. d8, d21, and d120 are day 8, day 21, and day 120, respectively. LM, littermate. (B) Histograms represent the mean percentages or numbers from two to three independent experiments by flow cytometric analysis. NS mix is a mixture of two different CD4+ T-cell-specific epitopes in the three-dimensional nonstructural protein (RNA polymerase) that are uniquely recognized by TMEV-infected SJL mice (B. S. Kang and B. S. Kim, unpublished observations). The proportion of epitope-specific IFN-γ-producing CD4+ T cells (top), total number of CNS-infiltrating CD4+ T cells (middle), and number of epitope-specific CD4+ T cells in the CNS (bottom) are shown. Statistical significance was analyzed by the Student's t test. *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
Capsid-specific CD8+ T-cell response in the CNS is tightly tolerized in B6 P1-Tg mice but not in SJL P1-Tg mice.
To determine whether capsid-specific CD8+ T-cell responses in the CNS of P1-Tg mice follow the CD4+ T-cell response pattern (Fig. 4), virus-specific CD8+ T-cell response levels were assessed by flow cytometry (Fig. 5). CD8+ T-cell responses to minor epitopes (VP3110-120 and VP3165-173) in the CNS were low (<5%) in both B6 P1-Tg mice and their littermates throughout the course of viral infection (data not shown), as previously shown for TMEV-infected B6 mice (36). Thus, only CD8+ T cells reactive to the predominant VP2121-130 epitope were further analyzed. Both the number of overall CNS-infiltrating CD8+ T cells and the proportion of IFN-γ-producing epitope-specific CD8+ T cells were greatly reduced (P < 0.005) in B6 P1-Tg mice during the course of viral infection (Fig. 5), leading to a severe reduction in the total number of epitope-specific CD8+ T cells. The levels of CD8+ T cells reactive to Db-VP2121-130 tetramers were similarly lower in B6 P1-Tg mice throughout the course of viral infection (Fig. 5B). These results indicate that a reduction in the levels of IFN-γ-producing virus-specific CD8+ T cells reflects the inability of B6 P1-Tg mice to induce the epitope-specific CD8+ T-cell response rather than their functional deficiency.
FIG. 5.
Epitope-specific IFN-γ-producing CD8+ T-cell levels in the CNS of TMEV-infected P1-Tg mice. (A) CNS-infiltrating cells from P1-Tg mice or their littermates were isolated at 8, 21, and 120 days pi and stimulated with the indicated peptides (2 μM) for 6 h. Cells were stained for both CD8 and intracellular IFN-γ, followed by flow cytometric analysis. The percentage of IFN-γ+ and CD8+ T cells is shown in the upper left quadrant of each plot. A representative flow cytometry plot from two to three independent experiments is shown. d8, d21, and d120 are day 8, day 21, and day 120, respectively. LM, littermate. CON represents control cultures similarly incubated with phosphate-buffered saline. (B) Proportion of epitope-specific CD8+ T cells (top rows of graphs for B6 and SJL mice), total number of CNS-infiltrating CD8+ T cells (second rows of graphs), and number of epitope-specific CD8+ T cells in the CNS are presented (third rows of graphs). The percentage of Db-VP2121-130 tetramer-staining CD8+ T cells among CNS-infiltrating cells of B6 mice also is presented (fourth row of the B6 graphs). Values given represent the mean percentages or numbers (±SD) of the results from three independent experiments. *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
In contrast to the drastic reduction (>80%) of VP2121-130-specific CD8+ T-cell response seen in B6 P1-Tg mice, only <40% of the predominant VP3159-166-specific CD8+ T-cell response was reduced in SJL P1-Tg mice at 8 days pi compared to that of their littermates, and it was reduced <30% thereafter compared to that of their littermates (Fig. 5B). Interestingly, CD8+ T-cell responses to subdominant epitopes in SJL P1-Tg mice were largely unaffected (Fig. 5B). In general, high-affinity CD8+ T cells are preferentially tolerized by deletion or anergy in vivo. In fact, the relative levels of IFN-γ-producing cells upon stimulation with serial 10-fold-decreased epitope peptide concentrations indicated that functional avidity to the dominant epitope (VP3159-166) is 50- and 100-fold higher than those to the subdominant epitopes (VP3173-181 and VP111-20, respectively) (data not shown). These results are consistent with the preferential reduction of high-affinity VP3159-166-specific CD8+ T cells over low-affinity subdominant epitope-reactive CD8+ T cells. Nevertheless, overall CNS-infiltrating CD8+ T-cell numbers were comparable between SJL P1-Tg mice and their littermates; the total numbers of virus-specific CD8+ T cells were not drastically different (Fig. 5B). Therefore, SJL mice likely are less efficient in censoring low-avidity autoreactive CD8+ T cells induced via molecular mimicry or polyclonal activation following viral infections.
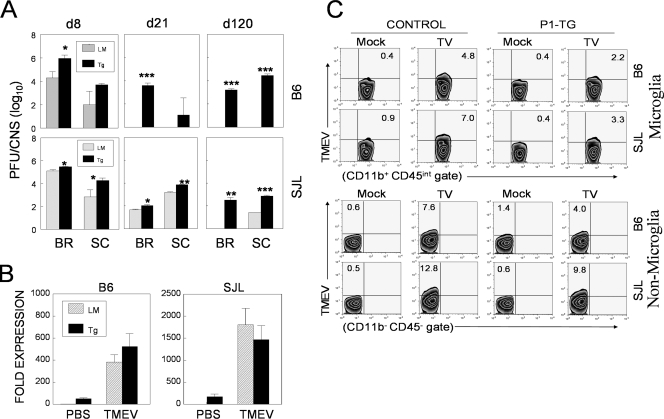
Viral loads are drastically increased in P1-Tg mice with both B6 and SJL backgrounds.
We next determined the effects of virus-specific immune tolerance in P1-Tg mice on viral persistence in the CNS. Both B6 and SJL P1-Tg mice displayed significant increases in viral persistence in the CNS throughout the course of viral infection compared to that of their control littermates. Most strikingly, B6 P1-Tg mice showed high levels of viral persistence at 120 days pi (P < 0.001), levels that were greater than those in susceptible SJL mice, while their littermates cleared viral persistence completely by 21 days pi (Fig. 6A). Similarly, viral persistence in the brain and spinal cord of SJL P1-Tg mice was significantly higher (3-fold to approximately 10-fold) at all time points (8, 21, and 120 days pi) compared to that of their littermates. These results strongly suggest that the immune tolerance to viral capsid determinants in P1-Tg mice with both backgrounds permits significantly increased viral persistence in the CNS, most drastically in B6 P1-Tg mice.
FIG. 6.
Increased viral persistence in the CNS of TMEV-infected P1-Tg mice. (A) The titers of the infectious virus in the CNS of TMEV-infected P1-Tg mice or their littermates with B6 and SJL backgrounds were determined by plaque assay at the indicated number of days pi. The PFU numbers represent the PFU/whole brain or spinal cord calculated from the pool of three to four mice per group. The statistical significance of the differences in infectious viral titers in the CNS was analyzed by the Student's t test. *, P < 0.05; **, P < 0.01; and ***, P < 0.001. d8, d21, and d120 are day 8, day 21, and day 120, respectively. PBS, phosphate-buffered saline. (B) Relative expression levels of VP1 RNA in the CNS cells (5 × 105/ml) infected in vitro (multiplicity of infection, 10) with TMEV for 24 h or left uninfected were assessed by real-time RT-PCR. Data shown were normalized to their GAPDH levels. Error bars indicate the SD of triplicate determinations. (C) The TMEV capsid antigen level was determined by intracellular staining at 48 h after TMEV infection (multiplicity of infection, 20).
However, it is conceivable that intact L protein produced in viral target cells of Tg mice promotes viral replication following TMEV infection by inhibiting the production of type I IFNs (53). To examine this possibility, we first compared P1 expression levels in vitro between CNS cells from naïve control and P1-Tg mice with/without infection (Fig. 6B). The results demonstrated that the levels of VP1 expression in uninfected P1-Tg cells were as much as 10-fold lower than those of infected littermate control cells in both strains. However, similar levels of VP1 were expressed in P1-Tg and control cells after infection with TMEV. Further flow cytometric analysis of capsid proteins showed that the levels of virus-infected P1-Tg cells were somewhat lower than those of infected littermate control cells, whereas uninfected P1-Tg cells failed to mount detectable levels of capsid proteins (Fig. 6C). Similar results were obtained when three-dimensional messages were assessed (data not shown). These results clearly demonstrated that there is no increased viral replication in CNS cells from Tg mice compared to that of control cells. Therefore, it is most likely that the enhanced viral loads in the CNS of TMEV-infected P1-Tg mice (Fig. 6A) reflect the deficiency in immune-mediated viral control due to the Tg tolerance to the capsid epitopes.
Viral persistence is not correlated with the development of demyelinating disease.
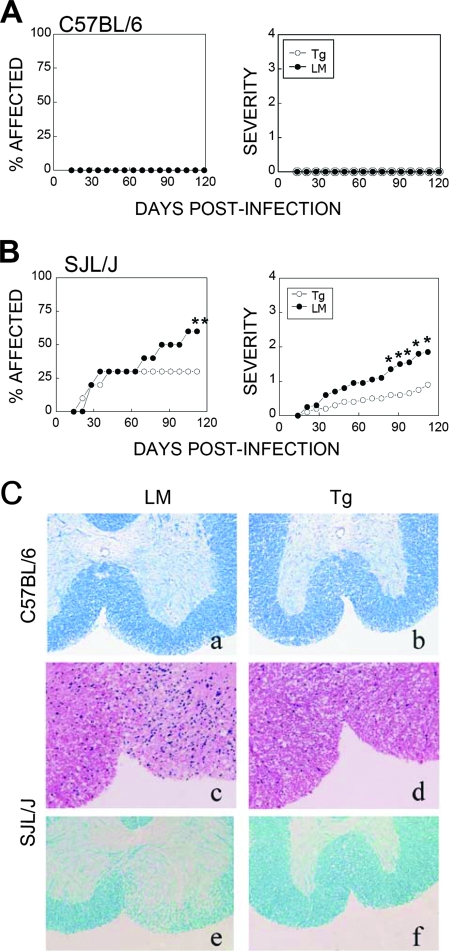
To further correlate viral persistence in the CNS with disease development levels in P1-Tg mice, demyelinating disease levels were assessed following infection with TMEV (Fig. 7). Neither B6 P1-Tg mice nor their littermates developed detectable clinical symptoms after infection with the virus (6 × 106 PFU/mouse); B6 P1-Tg mice remained free of symptomatic disease during the course (>120 days) of viral infection (Fig. 7A). The possibility that P1-Tg mice develop nonsymptomatic demyelinating lesions due to their inability to clear viruses in the CNS was further examined after staining the spinal cords with Luxol-fast blue (Fig. 7C). Despite extensive viral persistence in the CNS that exceeded the levels found in SJL mice, visible inflammation and demyelinating lesions were not seen as late as 120 days pi in B6 P1-Tg mice, similarly to their littermates (Fig. 7C). These data indicate that viral persistence is not sufficient for the induction of demyelination or demyelination-associated clinical symptoms in mice with the resistant B6 background.
FIG. 7.
Development of demyelination and clinical disease levels in TMEV-infected P1-Tg mice and their littermates (LM). The development of clinical symptoms was examined during 120 days after infection with TMEV strain BeAn 175R-K into B6 P1-Tg mice (n = 9) and their control littermates (n = 10) (A) or SJL P1-Tg mice (n = 25) and their littermates (n = 27) (B). Clinical symptoms were assessed weekly based on behavioral changes as described in Materials and Methods. The statistical significance of the percentage of affected SJL mice was tested by Fisher's exact test (P = 0.028 at 105 to 112 days pi), and clinical scores were analyzed by Mann-Whitney U tests. *, P < 0.05; **, P < 0.01. (C) Cross-sections of spinal cord from an infected B6 P1-Tg mouse (b) and a littermate (a) at 120 days pi were stained with Luxol-fast blue for myelin. The histologic examinations show normal-appearing white matter in the anterior columns of both animals. Cross-sections of spinal cord from an infected SJL P1-Tg mouse (d) and a littermate (c) stained with hematoxylin and eosin show rich inflammatory cell infiltration in the anterior column of the littermate but no inflammation in the Tg mouse. Serial sections from both animals stained with Luxol-fast blue show the striking pallor of the anterior-column white matter in the littermate (f) corresponding with the inflamed area, while no white-matter pallor is seen for the Tg animal (e). All sections were from paraffin-embedded spinal cords at 120 days pi. Magnification, ×200.
We expected that SJL P1-Tg mice develop more severe demyelinating disease. Therefore, we infected mice by design with a lower dose of TMEV (2 × 105 PFU/mouse) to detect the potential exacerbation of disease in Tg mice compared to that of the littermates. In SJL P1-Tg mice and their littermates infected with the same viral stock (175R-K), both disease incidence (P = 0.028 at 105 to 112 days pi) and severity (P < 0.05 after 84 days pi) in P1-Tg mice were significantly lower compared to those of their littermates (Fig. 7B), despite the increased viral persistence levels in the CNS of P1-Tg mice (Fig. 6). As the development of TMEV-IDD in susceptible SJL mice is thought to be associated with the loss of the myelin sheath, the levels of inflammatory cellular infiltration and demyelination were further assessed by histological examination at 120 days pi (Fig. 7C). Spinal cord cross-sections of SJL littermates stained with hematoxylin and eosin showed the hypercellularity of the spinal cord white matter, reflecting the presence of lymphoid cells and monocytes. Adjacent sections, stained with Luxol-fast blue, showed a corresponding pallor that reflected demyelination. In contrast, the white matter of spinal cords from representative relatively healthy SJL P1-Tg mice appeared normal. However, mild demyelination was observed in SJL P1-Tg mice that were clinically affected (data not shown). These results clearly indicate that SJL P1-Tg mice develop significantly reduced levels of symptomatic disease and demyelination compared to that of their littermates despite the higher level of viral persistence in the CNS (Fig. 6). These results strongly imply that the development of clinical disease correlates with demyelination levels, but not viral persistence levels, in mice with resistant as well as susceptible backgrounds.
DISCUSSION
Most epitopes recognized by CNS-infiltrating CD4+ and CD8+ T cells in infected B6 and SJL mice are located within the P1 region of the TMEV genome (Fig. 1). Therefore, it is feasible to induce virus-specific immune unresponsiveness by expressing the P1 region as Tg neoself proteins. If this is the case, P1-Tg mice may display high levels of viral persistence due to inefficient viral clearance resulting from the loss of virus-specific immune responses. In this experimental setting, the importance of virus-specific immune responses and viral persistence in the development of demyelinating disease can be investigated in conjunction with susceptible or resistant backgrounds. In this study, we generated both SJL and B6 P1-Tg mice by expressing the P1 region encoding the leader polypeptide and capsid proteins under the hCMV promoter, as previously used to express other transgenes (28, 52). In agreement with previous reports, the P1 transgene was expressed mainly in the brain and, to a lesser extent, in the liver (Fig. 1).
It is interesting that the tolerance of both B and T cells to P1 epitopes which are neoself antigens seems to be leaky in autoimmune-prone SJL mice (Fig. 3 to 5). Therefore, it is conceivable that self-reactive B- and T-cell clones are not properly deleted or anergized during maturation in SJL mice, whereas self tolerance in B6 mice is effectively maintained. This notion is consistent with previous reports demonstrating that autoimmune-prone SJL mice have a high precursor frequency of autoreactive PLP139-151-specific CD4+ T cells (1 to 40 cells per 20,000), possibly due to escape from central tolerance (1). Similarly, deficiency in central and/or peripheral tolerance in NOD mice has been implicated in the development of autoimmune insulitis and overt diabetes (29, 30, 58). These results strongly suggest the possibility that inflammatory responses following viral infection also differentially expand autoreactive T cells, which may be involved in TMEV-IDD in susceptible autoimmune-prone SJL mice (39). However, such T-cell expansion may require immune responses to viral epitopes, as the viral persistence level alone is not correlated with TMEV-IDD (Fig. 6).
The Tg expression of the TMEV P1 region resulted in a significant reduction of virus-specific T-cell and antibody responses in the CNS and periphery of P1-Tg mice with the resistant B6 background (Fig. 2 to 5) and led to viral persistence in the CNS (Fig. 6). In particular, the unresponsiveness of CD8+ T-cell responses to the predominant epitope was the most severe in the CNS compared to that for the periphery and TMEV-specific CD4+ T-cell and antibody responses (Fig. 3 to 5). The importance of CD8+ T cells in viral resolution is consistent with previous reports of mice with resistant backgrounds (38, 45, 47). However, it is also possible that deficiencies in virus-specific CD4+ T-cell and antibody responses in P1-Tg mice contribute to the lack of viral clearance, as these immune responses play a role in TMEV clearance from the CNS, especially during the early stages of viral infection (6, 22, 40, 43). Surprisingly, however, TMEV-infected B6 P1-Tg mice with high levels of viral persistence developed neither symptomatic disease nor demyelinating lesions as late as 250 days pi (Fig. 7A and data not shown). It is particularly interesting that the severe loss of virus-specific CD8+ T-cell responses in B6 P1-Tg mice did not result in demyelinating disease, whereas perforin- or β2-microglobulin-deficient mice with resistant H-2b backgrounds showed increased viral persistence and demyelination in the CNS as well as the development of a low but significant level of clinical disease (12, 47, 49). These results imply that NKT and/or other nonclassical class I-restricted CD8+ T-cell functions also play a protective role against demyelination and/or the development of TMEV-IDD. Alternatively, minor epitope (VP2165-173 and VP3110-120)-specific CD8+ T cells of the Tc-2 type may play a regulatory role, albeit with a smaller magnitude of induction (35). It was previously shown that resistant B6 mice, in the absence of both CD8+ T-cell and antibody responses, develop encephalitis rather than demyelination following infection with TMEV (22). Therefore, the presence of low but significant levels of anti-TMEV antibody responses in P1-Tg mice may have prevented the development of encephalitis. In addition, unknown antiviral immune responses to nonstructural proteins of TMEV also may contribute to the prevention of viral encephalitis. Nevertheless, our results clearly indicate that viral persistence alone is not sufficient to induce TMEV-IDD in B6 P1-Tg mice.
A group of investigators previously showed that B10 VP1-Tg mice are susceptible to TMEV-induced demyelination (31). These Tg mice displayed VP1-specific CD8+ T-cell tolerance after TMEV infection but similar levels of lymphocyte proliferation and virus-specific antibody responses after repeated immunizations with UV-TMEV. These investigators speculated that class II-dependent immune effector functions are largely unaffected by the Tg expression of TMEV proteins under the major histocompatibility complex (MHC) class I promoter. These findings are somewhat different from ours: we found that in B6 P1-Tg mice, the reduction in antibody responses was maintained until 120 days pi, although the CD4+ T-cell tolerance was detectable only until 21 days pi. In addition, our B6 P1-Tg mice did not exhibit detectable demyelination (Fig. 7) despite the highly elevated level of viral persistence in the CNS (Fig. 6). These discrepancies may be due in part to different immunological assessments and/or TMEV strains (DA versus BeAn) employed in these studies. Alternatively, differences in the genetic background between B10 and B6 mice, transgene expression patterns of the promoters used, and the size and site of TMEV genome inserts may have contributed to these discrepancies. Nevertheless, the tolerance levels of CD4+ T-cell responses to viral epitopes, particularly proliferative responses, are transient only compared to their cytokine production and virus-specific CD8+ T responses in both B6 and SJL P1-Tg mice expressed under the hCMV promoter (Fig. 1 to 5). The mechanisms involved in such differential maintenance of tolerance between virus-specific CD8+ and CD4+ T cells in P1-Tg mice are unclear at this time, but they appear to be unrelated to the expression promoters.
Viral persistence has been considered a critical susceptibility factor of TMEV-IDD, particularly in susceptible mice (2, 4, 33). It was previously reported that infectious viral titers in the CNS of different mouse strains do not correlate with levels of clinical disease (8). However, these results are difficult to interpret, since immune-associated or nonimmune-associated genes in different mouse strains also are likely to influence the outcome of TMEV-IDD (7, 24, 34). Interestingly, the expression of H-2Db in susceptible FVB (H-2q) mice renders them resistant, but this resistance is lost when these FVB/Db Tg mice are tolerized by a soluble VP2121-130 peptide infusion (3, 38) or the Tg expression of either the whole VP2 protein or the VP2121-130 CD8+ T-cell epitope region (46). These tolerized Tg mice exhibited viral persistence in the CNS and developed chronic demyelinating disease. However, the focus of these studies was on analyzing the role of introduced virus-specific, H-2Db-restricted, predominant CD8+ T-cell responses of resistant mice expressed in susceptible mice. Therefore, the role of viral persistence in the pathogenesis of demyelination with the native immune system in susceptible mice remained unclear.
In contrast to the above-described studies with FVB/Db Tg approaches, we expressed the TMEV P1 transgene in highly susceptible SJL mice in order to investigate the association between viral persistence and immunological responses involved in the pathogenesis of demyelinating disease (Fig. 1). Surprisingly, SJL P1-Tg mice, which exhibited elevated viral loads in the brain and/or spinal cord throughout the course of viral infection, displayed significantly lower levels of demyelination, disease incidence, and severity than control mice (Fig. 7). These results clearly demonstrate that a higher level of viral persistence alone does not lead to more severe demyelinating disease, and the level of anticapsid immune responses is a critical factor for pathogenesis in susceptible SJL mice. Perhaps the reduced T-cell responses to P1 antigens include a T-cell population involved in the pathogenesis of demyelination, resulting in increased viral persistence but reduced pathogenesis. This notion is consistent with findings from previous studies that suggest the pathogenic potential of viral capsid-specific CD4+ T cells (16, 57). However, the differences in the antiviral CD4+ T-cell responses in SJL P1-Tg mice become minimal during or following the onset of demyelinating disease, suggesting that the role of CD4+ T cells in pathogenesis is limited. On the other hand, CD8+ T-cell levels specific for the predominant epitope, VP3159-166, were significantly reduced in SJL P1-Tg mice, although CD8+ T-cell levels specific for subdominant epitopes (VP3173-181 and VP111-20) were largely unaffected throughout the course of infection (Fig. 5). Therefore, it is conceivable that VP3159-166-specific CD8+ T cells are involved in the pathogenesis of TMEV-IDD. This possibility is seemingly inconsistent with a previous report demonstrating increased demyelinating disease in β2-microglobin-deficient SJL mice (4). However, the differences between the selective elimination of CD8+ T cells in this P1-Tg system and the global unresponsiveness of all T cells restricted by MHC class I or MHC class I-like molecules associated with β2-microglobin in β2-microglobin-deficient SJL mice may result in such discrepancies.
It is very unlikely that L protein is functionally expressed in Tg cells, because there is no viral protease associated with the L protein in the P1-Tg construct. In addition, we have previously demonstrated that antigen-presenting cells (macrophages and dendritic cells) as well as microglia from susceptible SJL mice produce higher levels of type I IFNs, yet the viral replication levels are severalfold higher than those from resistant B6 mice (17, 18). These results indicate that TMEV BeAn infection strongly induces type I IFNs in the presence of L protein and that viral replication is not interrupted in cells producing type I IFNs. Moreover, our results (Fig. 6B, C) clearly showed that there is no increased replication in cells from Tg mice after in vitro infection. Therefore, the increased viral loads in the CNS of Tg mice (Fig. 6A) likely reflect the deficient anticapsid immunity levels due to the P1-Tg expression.
We have utilized TMEV P1-Tg mice with resistant and susceptible backgrounds in order to induce specific immune tolerance toward viral capsid antigens as autoantigens. Our results reveal that viral persistence levels are not associated with the development of TMEV-IDD in mice with either resistant or susceptible backgrounds. CNS T-cell response levels against certain viral capsid antigens appear to be critical for the pathogenesis of demyelination. In addition, tolerance to viral autoantigens in Tg mice is poorly maintained in autoimmune-prone SJL mice, suggesting deficiencies in maintaining the self-tolerance of T cells, particularly in the CD4+ T-cell compartment and low-avidity CD8+ T cells reactive to subdominant epitopes. Such deficiencies in maintaining self-tolerance also may contribute to the pathogenesis of virally induced demyelinating disease in susceptible mice, as viral infection-induced inflammatory responses may expand autoreactive T cells nonspecifically in the inflammatory environment or specifically via molecular mimicry (39). Further studies utilizing Tg mice with the susceptible background carrying specific tolerance to more restricted viral determinants are likely to shed light on the pathogenic mechanisms of epitope-specific T cells involved in the development of immune-mediated demyelinating disease.
Acknowledgments
This work was supported by U.S. Public Health Service grants (RO1 NS28752, RO1 NS33008, and PO1 NS23349) and by a grant from the National Multiple Sclerosis Society (RG 3392-A5).
Footnotes
Published ahead of print on 19 March 2008.
REFERENCES
- 1.Anderson, A. C., L. B. Nicholson, K. L. Legge, V. Turchin, H. Zaghouani, and V. K. Kuchroo. 2000. High frequency of autoreactive myelin proteolipid protein-specific T cells in the periphery of naive mice: mechanisms of selection of the self-reactive repertoire. J. Exp. Med. 191761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aubagnac, S., M. Brahic, and J. F. Bureau. 1999. Viral load and a locus on chromosome 11 affect the late clinical disease caused by Theiler's virus. J. Virol. 737965-7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azoulay, A., M. Brahic, and J.-F. Bureau. 1994. FVB mice transgenic for the H-2Db gene become resistant to persistent infection by Theiler's virus. J. Virol. 684049-4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Begolka, W. S., L. M. Haynes, J. K. Olson, J. Padilla, K. L. Neville, M. Dal Canto, J. Palma, B. S. Kim, and S. D. Miller. 2001. CD8-deficient SJL mice display enhanced susceptibility to Theiler's virus infection and increased demyelinating pathology. J. Neurovirol. 7409-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrow, P., P. Tonks, C. J. Welsh, and A. A. Nash. 1992. The role of CD8+ T cells in the acute and chronic phases of Theiler's murine encephalomyelitis virus-induced disease in mice. J. Gen. Virol. 731861-1865. [DOI] [PubMed] [Google Scholar]
- 6.Borrow, P., C. J. Welsh, and A. A. Nash. 1993. Study of the mechanisms by which CD4+ T cells contribute to protection in Theiler's murine encephalomyelitis. Immunology 80502-506. [PMC free article] [PubMed] [Google Scholar]
- 7.Brahic, M., J. F. Bureau, and T. Michiels. 2005. The genetics of the persistent infection and demyelinating disease caused by Theiler's virus. Annu. Rev. Microbiol. 59279-298. [DOI] [PubMed] [Google Scholar]
- 8.Clatch, R. J., H. L. Lipton, and S. D. Miller. 1987. Class II-restricted T cell responses in Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease. II. Survey of host immune responses and central nervous system virus titers in inbred mouse strains. Microb. Pathog. 3327-337. [DOI] [PubMed] [Google Scholar]
- 9.Crane, M. A., R. Yauch, M. C. Dal Canto, and B. S. Kim. 1993. Effect of immunization with Theiler's virus on the course of demyelinating disease. J. Neuroimmunol. 4567-73. [DOI] [PubMed] [Google Scholar]
- 10.Dal Canto, M. C., B. S. Kim, S. D. Miller, and R. W. Melvold. 1996. Theiler's murine encephalomyelitis virus (TMEV)-induced demyelination: a model for human multiple sclerosis. Methods 10453-461. [DOI] [PubMed] [Google Scholar]
- 11.Dethlefs, S., N. Escriou, M. Brahic, S. van der Werf, and E. L. Larsson-Sciard. 1997. Theiler's virus and Mengo virus induce cross-reactive cytotoxic T lymphocytes restricted to the same immunodominant VP2 epitope in C57BL/6 mice. J. Virol. 715361-5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiette, L., C. Aubert, M. Brahic, and C. P. Rossi. 1993. Theiler's virus infection of β2-microglobulin-deficient mice. J. Virol. 67589-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedmann, A., G. Frankel, Y. Lorch, and L. Steinman. 1987. Monoclonal anti-I-A antibody reverses chronic paralysis and demyelination in Theiler's virus-infected mice: critical importance of timing of treatment. J. Virol. 61898-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuller, A. C., B. Kang, H. K. Kang, H. Yahikozowa, M. C. Dal Canto, and B. S. Kim. 2005. Gender bias in Theiler's virus-induced demyelinating disease correlates with the level of antiviral immune responses. J. Immunol. 1753955-3963. [DOI] [PubMed] [Google Scholar]
- 15.Gerety, S. J., R. J. Clatch, H. L. Lipton, R. G. Goswami, M. K. Rundell, and S. D. Miller. 1991. Class II-restricted T cell responses in Theiler's murine encephalomyelitis virus-induced demyelinating disease. IV. Identification of an immunodominant T cell determinant on the N-terminal end of the VP2 capsid protein in susceptible SJL/J mice. J. Immunol. 1462401-2408. [PubMed] [Google Scholar]
- 16.Gerety, S. J., M. K. Rundell, M. C. Dal Canto, and S. D. Miller. 1994. Class II-restricted T cell responses in Theiler's murine encephalomyelitis virus-induced demyelinating disease. VI. Potentiation of demyelination with and characterization of an immunopathologic CD4+ T cell line specific for an immunodominant VP2 epitope. J. Immunol. 152919-929. [PubMed] [Google Scholar]
- 17.Hou, W., E. Y. So, and B. S. Kim. 2007. Role of dendritic cells in differential susceptibility to viral demyelinating disease. PLoS Pathog. 3e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin, Y. H., M. Mohindru, M. H. Kang, A. C. Fuller, B. Kang, D. Gallo, and B. S. Kim. 2007. Differential virus replication, cytokine production, and antigen-presenting function by microglia from susceptible and resistant mice infected with Theiler's virus. J. Virol. 8111690-11702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang, B., H. K. Kang, and B. S. Kim. 2005. Identification of capsid epitopes of Theiler's virus recognized by CNS-infiltrating CD4(+) T cells from virus-infected C57BL/6 mice. Virus Res. 10857-61. [DOI] [PubMed] [Google Scholar]
- 20.Kang, B. S., M. A. Lyman, and B. S. Kim. 2002. Differences in avidity and epitope recognition of CD8+ T cells infiltrating the central nervous systems of SJL/J mice infected with BeAn and DA strains of Theiler's murine encephalomyelitis virus. J. Virol. 7611780-11784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang, B. S., M. A. Lyman, and B. S. Kim. 2002. The majority of infiltrating CD8+ T cells in the central nervous system of susceptible SJL/J mice infected with Theiler's virus are virus specific and fully functional. J. Virol. 766577-6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang, B. S., J. P. Palma, M. A. Lyman, M. Dal Canto, and B. S. Kim. 2005. Antibody response is required for protection from Theiler's virus-induced encephalitis in C57BL/6 mice in the absence of CD8(+) T cells. Virology 34084-94. [DOI] [PubMed] [Google Scholar]
- 23.Kang, B. S., H. Yahikozawa, C. S. Koh, and B. S. Kim. 2007. Oral administration of live virus protects susceptible mice from developing Theiler's virus-induced demyelinating disease. Virology 366185-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kappel, C. A., M. C. Dal Canto, R. W. Melvold, and B. S. Kim. 1991. Hierarchy of effects of the MHC and T cell receptor b-chain genes in susceptibility to Theiler's murine encephalomyelitis virus-induced demyelinating disease. J. Immunol. 1474322-4326. [PubMed] [Google Scholar]
- 25.Kim, B. S., M. A. Lyman, B. S. Kang, H. K. Kang, H. G. Lee, M. Mohindru, and J. P. Palma. 2001. Pathogenesis of virus-induced immune-mediated demyelination. Immunol. Res. 24121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim, B. S., J. P. Palma, D. Kwon, and A. C. Fuller. 2005. Innate immune response induced by Theiler's murine encephalomyelitis virus infection. Immunol. Res. 311-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim, B. S., R. L. Yauch, Y. Y. Bahk, J. A. Kang, M. C. Dal Canto, and C. K. Hall. 1998. A spontaneous low-pathogenic variant of Theiler's virus contains an amino acid substitution within the predominant VP1233-250 T-cell epitope. J. Virol. 721020-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koedood, M., A. Fichtel, P. Meier, and P. J. Mitchell. 1995. Human cytomegalovirus (HCMV) immediate-early enhancer/promoter specificity during embryogenesis defines target tissues of congenital HCMV infection. J. Virol. 692194-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kreuwel, H. T., J. A. Biggs, I. M. Pilip, E. G. Pamer, D. Lo, and L. A. Sherman. 2001. Defective CD8+ T cell peripheral tolerance in nonobese diabetic mice. J. Immunol. 1671112-1117. [DOI] [PubMed] [Google Scholar]
- 30.Lesage, S., S. B. Hartley, S. Akkaraju, J. Wilson, M. Townsend, and C. C. Goodnow. 2002. Failure to censor forbidden clones of CD4 T cells in autoimmune diabetes. J. Exp. Med. 1961175-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin, X., M. K. Njenga, A. J. Johnson, K. D. Pavelko, C. S. David, L. R. Pease, and M. Rodriguez. 2002. Transgenic expression of Theiler's murine encephalomyelitis virus genes in H-2b mice inhibits resistance to virus-induced demyelination. J. Virol. 767799-7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipton, H. L., and M. C. Dal Canto. 1976. Theiler's virus-induced demyelination: prevention by immunosuppression. Science 19262-64. [DOI] [PubMed] [Google Scholar]
- 33.Lipton, H. L., A. S. Kumar, and M. Trottier. 2005. Theiler's virus persistence in the central nervous system of mice is associated with continuous viral replication and a difference in outcome of infection of infiltrating macrophages versus oligodendrocytes. Virus Res. 111214-223. [DOI] [PubMed] [Google Scholar]
- 34.Lipton, H. L., and R. Melvold. 1984. Genetic analysis of susceptibility to Theiler's virus-induced demyelinating disease in mice. J. Immunol. 1321821-1825. [PubMed] [Google Scholar]
- 35.Lyman, M. A., H. G. Lee, B. S. Kang, H. K. Kang, and B. S. Kim. 2002. Capsid-specific cytotoxic T lymphocytes recognize three distinct H-2Db-restricted regions of the BeAn strain of Theiler's virus and exhibit different cytokine profiles. J. Virol. 763125-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyman, M. A., J. Myoung, M. Mohindru, and B. S. Kim. 2004. Quantitative, not qualitative, differences in CD8(+) T cell responses to Theiler's murine encephalomyelitis virus between resistant C57BL/6 and susceptible SJL/J mice. Eur. J. Immunol. 342730-2739. [DOI] [PubMed] [Google Scholar]
- 37.Martin, R. M., J. L. Brady, and A. M. Lew. 1998. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J. Immunol. Methods 212187-192. [DOI] [PubMed] [Google Scholar]
- 38.Mendez-Fernandez, Y. V., A. J. Johnson, M. Rodriguez, and L. R. Pease. 2003. Clearance of Theiler's virus infection depends on the ability to generate a CD8+ T cell response against a single immunodominant viral peptide. Eur. J. Immunol. 332501-2510. [DOI] [PubMed] [Google Scholar]
- 39.Miller, S. D., C. L. Vanderlugt, W. S. Begolka, W. Pao, R. L. Yauch, K. L. Neville, Y. Katz-Levy, A. Carrizosa, and B. S. Kim. 1997. Persistent infection with Theiler's virus leads to CNS autoimmunity via epitope spreading. Nat. Med. 31133-1136. [DOI] [PubMed] [Google Scholar]
- 40.Mohindru, M., B. Kang, and B. S. Kim. 2006. Initial capsid-specific CD4(+) T cell responses protect against Theiler's murine encephalomyelitisvirus-induced demyelinating disease. Eur. J. Immunol. 362106-2115. [DOI] [PubMed] [Google Scholar]
- 41.Murray, P. D., D. B. McGavern, X. Lin, M. K. Njenga, J. Leibowitz, L. R. Pease, and M. Rodriguez. 1998. Perforin-dependent neurologic injury in a viral model of multiple sclerosis. J. Neurosci. 187306-7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murray, P. D., K. D. Pavelko, J. Leibowitz, X. Lin, and M. Rodriguez. 1998. CD4+ and CD8+ T cells make discrete contributions to demyelination and neurologic disease in a viral model of multiple sclerosis. J. Virol. 727320-7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Njenga, M. K., K. D. Pavelko, J. Baisch, X. Lin, C. David, J. Leibowitz, and M. Rodriguez. 1996. Theiler's virus persistence and demyelination in major histocompatibility complex class II-deficient mice. J. Virol. 701729-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oleszak, E. L., J. R. Chang, H. Friedman, C. D. Katsetos, and C. D. Platsoucas. 2004. Theiler's virus infection: a model for multiple sclerosis. Clin. Microbiol. Rev. 17174-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palma, J. P., H. G. Lee, M. Mohindru, B. S. Kang, M. Dal Canto, S. D. Miller, and B. S. Kim. 2001. Enhanced susceptibility to Theiler's virus-induced demyelinating disease in perforin-deficient mice. J. Neuroimmunol. 116125-135. [DOI] [PubMed] [Google Scholar]
- 46.Pavelko, K. D., L. R. Pease, C. S. David, and M. Rodriguez. 2007. Genetic deletion of a single immunodominant T-cell response confers susceptibility to virus-induced demyelination. Brain Pathol. 17184-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pullen, L. C., S. D. Miller, M. C. Dal Canto, and B. S. Kim. 1993. Class I-deficient resistant mice intracerebrally inoculated with Theiler's virus show an increased T cell response to viral antigens and susceptibility to demyelination. Eur. J. Immunol. 232287-2293. [DOI] [PubMed] [Google Scholar]
- 48.Pullen, L. C., S. H. Park, S. D. Miller, M. C. Dal Canto, and B. S. Kim. 1995. Treatment with bacterial LPS renders genetically resistant C57BL/6 mice susceptible to Theiler's virus-induced demyelinating disease. J. Immunol. 1554497-4503. [PubMed] [Google Scholar]
- 49.Rodriguez, M., A. J. Dunkel, R. L. Thiemann, J. Leibowitz, M. Zijlstra, and R. Jaenisch. 1993. Abrogation of resistance to Theiler's virus-induced demyelination in H-2b mice deficient in beta 2-microglobulin. J. Immunol. 151266-276. [PubMed] [Google Scholar]
- 50.Rodriguez, M., W. P. Lafuse, J. Leibowitz, and C. S. David. 1986. Partial suppression of Theiler's virus-induced demyelination in vivo by administration of monoclonal antibodies to immune-response gene products (Ia antigens). Neurology 36964-970. [DOI] [PubMed] [Google Scholar]
- 51.Schlitt, B. P., M. Felrice, M. L. Jelachich, and H. L. Lipton. 2003. Apoptotic cells, including macrophages, are prominent in Theiler's virus-induced inflammatory, demyelinating lesions. J. Virol. 774383-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmidt, E. V., G. Christoph, R. Zeller, and P. Leder. 1990. The cytomegalovirus enhancer: a pan-active control element in transgenic mice. Mol. Cell. Biol. 104406-4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Pesch, V., O. van Eyll, and T. Michiels. 2001. The leader protein of Theiler's virus inhibits immediate-early alpha/beta interferon production. J. Virol. 757811-7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yahikozawa, H., A. Inoue, C. S. Koh, Y. K. Choe, and B. S. Kim. 1997. Major linear antibody epitopes and capsid proteins differentially induce protective immunity against Theiler's virus-induced demyelinating disease. J. Virol. 713105-3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yauch, R. L., K. Kerekes, K. Saujani, and B. S. Kim. 1995. Identification of a major T-cell epitope within VP3 amino acid residues 24 to 37 of Theiler's virus in demyelination-susceptible SJL/J mice. J. Virol. 697315-7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yauch, R. L., and B. S. Kim. 1994. A predominant viral epitope recognized by T cells from the periphery and demyelinating lesions of SJL/J mice infected with Theiler's virus is located within VP1(233-244). J. Immunol. 1534508-4519. [PubMed] [Google Scholar]
- 57.Yauch, R. L., J. P. Palma, H. Yahikozawa, C. S. Koh, and B. S. Kim. 1998. Role of individual T-cell epitopes of Theiler's virus in the pathogenesis of demyelination correlates with the ability to induce a Th1 response. J. Virol. 726169-6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zucchelli, S., P. Holler, T. Yamagata, M. Roy, C. Benoist, and D. Mathis. 2005. Defective central tolerance induction in NOD mice: genomics and genetics. Immunity 22385-396. [DOI] [PubMed] [Google Scholar]