Abstract
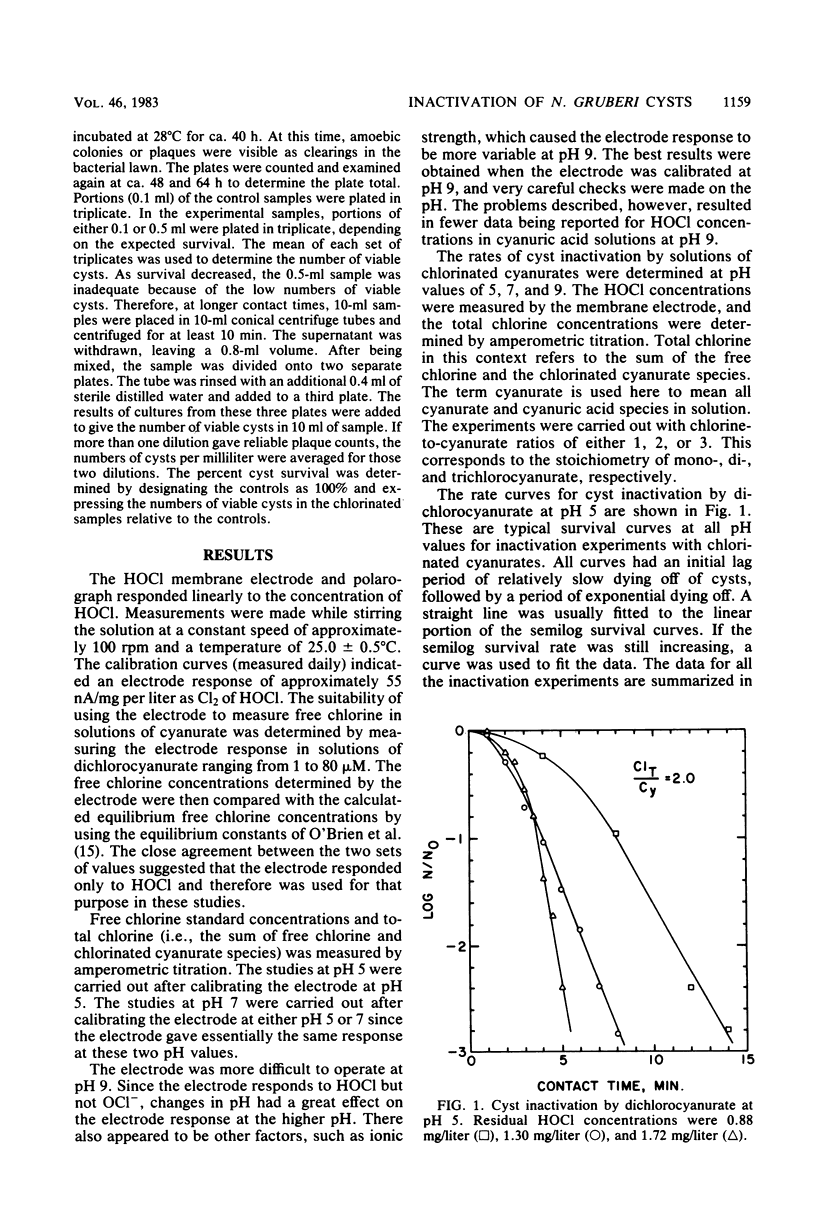
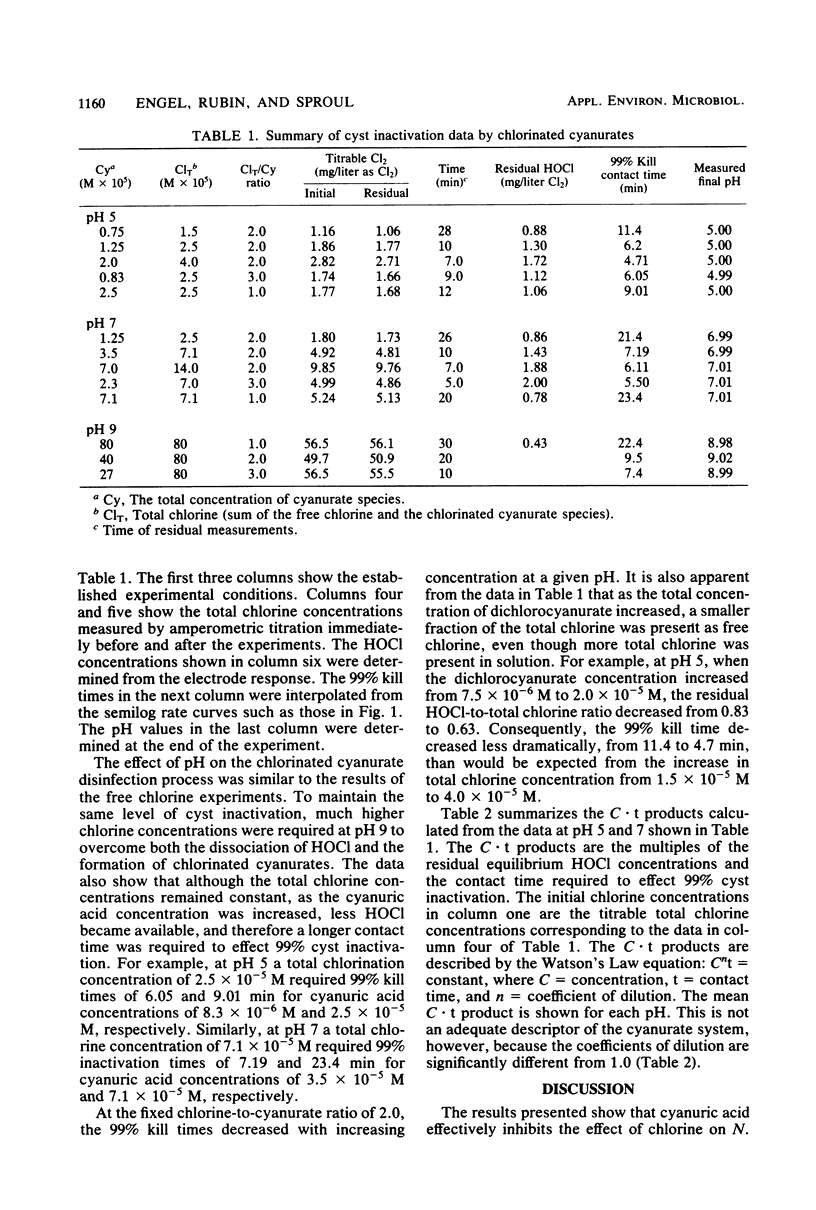
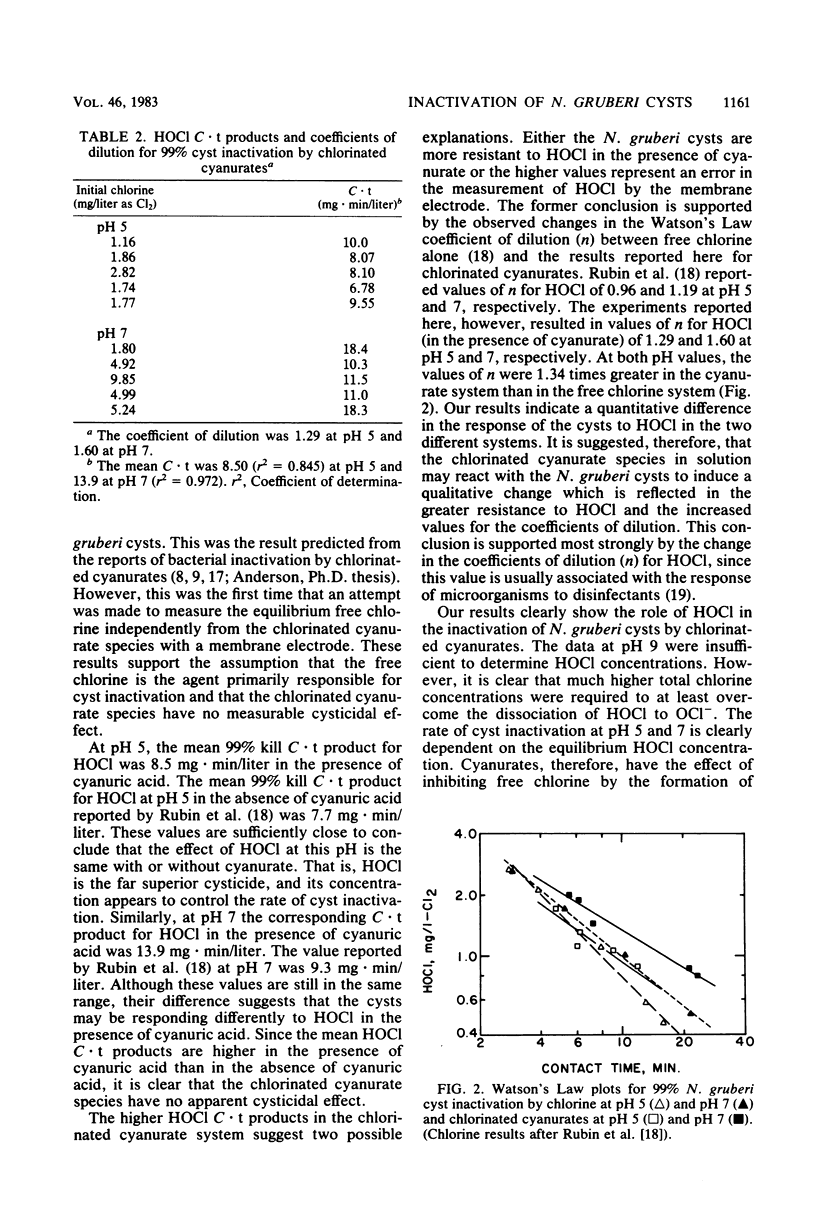
The resistance of Naegleria gruberi cysts to chlorine in the presence of cyanuric acid was compared at pH 5 and 7. An amperometric membrane electrode was used to measure HOCl concentrations independently of the chlorinated cyanurate species, thus permitting an analysis of the role of free chlorine versus chlorinated cyanurates in cyst inactivation. In the presence of cyanuric acid, the products of the HOCl residual and the contact time required for 99% cyst inactivation were 8.5 mg . min/liter and 13.9 mg . min/liter at pH 5 and 7, respectively. The Watson's Law coefficients of dilution (n) were 1.3 and 1.6 at pH 5 and 7, respectively. The results strongly suggest that HOCl is the predominant cysticide with no measurable cysticidal effect of the chlorinated cyanurate species.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K., Jamieson A. Primary amoebic meningoencephalitis. Lancet. 1972 Apr 22;1(7756):902–903. doi: 10.1016/s0140-6736(72)90772-6. [DOI] [PubMed] [Google Scholar]
- Chang S. L. Resistance of pathogenic Naegleria to some common physical and chemical agents. Appl Environ Microbiol. 1978 Feb;35(2):368–375. doi: 10.1128/aem.35.2.368-375.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cursons R. T., Brown T. J., Keys E. A. Effect of disinfectants on pathogenic free-living amoebae: in axenic conditions. Appl Environ Microbiol. 1980 Jul;40(1):62–66. doi: 10.1128/aem.40.1.62-66.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jonckheere J., van de Voorde H. Differences in destruction of cysts of pathogenic and nonpathogenic Naegleria and Acanthamoeba by chlorine. Appl Environ Microbiol. 1976 Feb;31(2):294–297. doi: 10.1128/aem.31.2.294-297.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duma R. J., Ferrell H. W., Nelson E. C., Jones M. M. Primary amebic meningoencephalitis. N Engl J Med. 1969 Dec 11;281(24):1315–1323. doi: 10.1056/NEJM196912112812401. [DOI] [PubMed] [Google Scholar]
- Fitzgerald G. P., DerVartanian M. E. Factors influencing the effectiveness of swimming pool bactericides. Appl Microbiol. 1967 May;15(3):504–509. doi: 10.1128/am.15.3.504-509.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald G. P., DerVartanian M. E. Pseudomonas aeruginosa for the evaluation of swimming pool chlorination and algicides. Appl Microbiol. 1969 Mar;17(3):415–421. doi: 10.1128/am.17.3.415-421.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadlec V., Cerva L., Skvárová J. Virulent Naegleria fowleri in an indoor swimming pool. Science. 1978 Sep 15;201(4360):1025–1025. doi: 10.1126/science.684423. [DOI] [PubMed] [Google Scholar]
- Robinton E. D., Mood E. W. An evaluation of the inhibitory influence of cyanuric acid upon swimming pool disinfection. Am J Public Health Nations Health. 1967 Feb;57(2):301–310. doi: 10.2105/ajph.57.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]


