Abstract
Isoprostanes (iPs) are free radical catalyzed prostaglandin isomers. Analysis of individual isomers of PGF2α—F2-iPs—in urine has reflected lipid peroxidation in humans. However, up to 64 F2-iPs may be formed, and it is unknown whether coordinate generation, disposition, and excretion of F2-iPs occurs in humans. To address this issue, we developed methods to measure individual members of the four structural classes of F2-iPs, using liquid chromatography/tandem mass spectrometry (LC/MS/MS), in which sample preparation is minimized. Authentic standards of F2-iPs of classes III, IV, V, and VI were used to identify class-specific ions for multiple reaction monitoring. Using iPF2α-VI as a model compound, we demonstrated the reproducibility of the assay in human urine. Urinary levels of all F2-iPs measured were elevated in patients with familial hypercholesterolemia. However, only three of eight F2-iPs were elevated in patients with congestive heart failure, compared with controls. Paired analyses by GC/MS and LC/MS/MS of iPF2α-VI in hypercholesterolemia and of 8,12-iso-iPF2α-VI in congestive heart failure were highly correlated. This approach will permit high throughput analysis of multiple iPs in human disease.
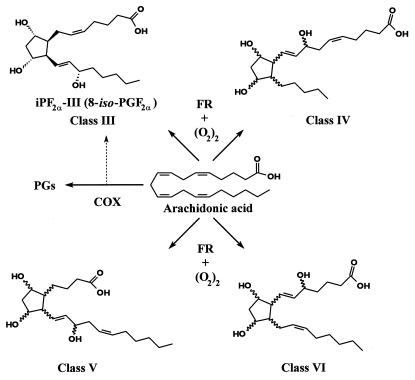
Isoeicosanoids are free radical catalyzed products of arachidonic acid. They are isomers of enzymatically formed prostaglandins, leukotrienes, and epoxyeicosatrienoic acids (1, 2). Unlike the enzymatic products of arachidonic acid, they are formed initially in situ in the phospholipid domain of cell membranes, from which they are cleaved by phospholipases (3), circulate in esterified and unesterified forms, and are excreted in urine. Several prostaglandin isomers—isoprostanes (iPs)—express biological activities in vitro (4–6) and may act as incidental ligands at membrane receptors for prostanoids and at peroxisome proliferator-activated receptors in the nucleus (7–9). Various assays for individual iPs have been developed (10–15), as these compounds have attraction as indices of lipid peroxidation in vivo. Although this approach has proven useful in selection of disease targets and rational dose finding for antioxidants (16–18), it is unknown how a single iP might reflect alterations in generation of this complex family of lipids. For example, in the case of isomers of PGF2α (F2-iPs), up to 64 compounds in four structural classes may be formed (19–21). It is unknown whether their relative predominance might differ as a function of their site of formation, their affinity for phospholipase cleavage, their metabolism, or their clearance from plasma into urine. Additionally, in the specific case of F2-iPs, the compound most frequently selected for analysis—iPF2α-III (formerly known as 8-iso-PGF2α)—may also be formed by the prostaglandin G/H synthase [cyclooxygenase (COX)] isozymes (10, 22). Because COX activation and oxidant stress may coincide (23–27), analysis of other F2-iPs, not formed by COX, has been necessary to interpret alterations in iPF2α-III generation (28, 29).
To begin to address these issues in human biology, we have developed methods that permit simultaneous analysis of selected members of each of the four classes of F2-iPs, using high performance liquid chromatography/electrospray ionization/tandem mass spectrometry (HPLC/ESI/MS/MS). By using homologous internal standards, it is possible to discriminate many individual isomers within a single chromatographic separation, bypassing much of the sample preparation associated with assays based on gas chromatography/mass spectrometry (GC/MS). Using this approach, we demonstrate that although urinary excretion of members of all four classes of the F2-iPs are increased in patients with hypercholesterolemia, only selected F2-iPs were significantly increased in patients with congestive cardiac failure. HPLC/ESI/MS/MS permits a more comprehensive assessment of iP generation in human diseases thought to be associated with increased lipid peroxidation in vivo.
Materials and Methods
Reagents.
Synthetic iPF2α-III was purchased from Cayman Chemicals (Ann Arbor, MI) and was used without further purification. We utilized HPLC-grade water (Milli-Q water purification system, Millipore) in the preparation of all aqueous solutions and mobile phases. HPLC-grade acetonitrile was purchased from J. T. Baker. HPLC-grade methanol and reagent-grade acetic acid were purchased from Fisher. HPLC-grade ammonium hydroxide and reagent-grade potassium hydroxide were purchased from Mallinckrodt. Reagent-grade 6 M hydrochloric acid was purchased from LabChem (Pittsburgh). Methanol-D (99.5 atom% D) and deuterium oxide (99.9 atom% D) were purchased from Aldrich. We obtained 190 proof ethanol from Pharmco Products (Brookfield, CT). Disodium hydrogen phosphate and potassium dihydrogen phosphate were purchased from Merck. The Sorensen buffer used for solid phase extraction consisted of 1/15 M potassium dihydrogen phosphate and 1/15 M disodium hydrogen phosphate. Standards of iPF2α-VI, [2H4]iPF2α-VI, iPF2α-V, and iPF2α-IV were synthesized according to previously published methods (30–32).
Isotope Exchange of Synthetic Isoprostanes.
Deuterium exchange of labile hydroxyl and carboxyl protons of the iP molecules was performed by dissolving 1 μg of iP in 100 μl of CH3OD, evaporating under a gentle nitrogen stream, and redissolving the sample in 100 μl of CH3OD:D2O (50:50). The deuterium exchanged samples were infused into the electrospray source of the mass spectrometer described below, using CH3OD:D2O (50:50) as the mobile phase. Exchange of carboxylate oxygens with 18O was performed as described (33).
Urine Sample Preparation.
Internal standards for iPF2α-III and iPF2α-VI were added to 1 ml of urine and were allowed to equilibrate for 15 min. Then, 0.5 ml of 15% KOH was added, and the sample was mixed and allowed to stand for 30 min. The pH was then adjusted with 6 M HCl to ≈2.5. The sample was then subjected to solid phase extraction (SPE). BondElut LMS polymer 100-mg SPE cartridges were purchased from Varian. The SPE cartridge was conditioned with 1 ml of ethanol and 1 ml of Sorensen buffer. The sample was applied to the cartridge, which was then sequentially washed with 1 ml each of Sorensen buffer and 5% ethanol in water. The analyte and internal standards were eluted from the cartridge by using 1 ml of ethanol. The eluate was collected and dried under a gentle stream of nitrogen. The resulting residue was then reconstituted with 100 μl of 20% acetonitrile in water and was filtered by centrifugation. The 0.2-μm Nylon microspin filters were purchased from Alltech Associates.
High Performance Liquid Chromatography.
The HPLC included an ABI 140B dual syringe pump (Applied Biosystems) and a Hypersil ODS-BD 3 μm, 2.0- × 150-mm column (Phenomenex, Torrance, CA). The mobile phase consisted of water (solvent A) and acetonitrile:methanol (95:5, solvent B), both with 0.005% acetic acid adjusted to pH 5.7 with ammonium hydroxide. The flow rate was controlled at 200 μl/min. The separation was carried out with a linear solvent gradient program starting at 25% B and ramping to 35% B in 20 min.
Mass Spectrometry.
A Micromass Quattro II mass spectrometer (Micromass, Beverly, MA) equipped with a coaxial electrospray source and triple quadrupole analyzer was used in these studies. The HPLC eluate was split from 200 μl/min to 50 μl/min before entering the electrospray source. The instrument was operated in the negative ion mode with the capillary voltage set to 3 kV, the sampling cone voltage to 40 V, and the source temperature to 70°C. The argon gas pressure in the radiofrequency-only region was set to 2 microbars (1 bar = 100 kPa). The collision energy was 23 eV. The analyzers were set in the multiple reaction monitoring mode.
Method Evaluation.
The linearity of the assay was demonstrated by spiking different concentrations (1.0, 2.0, 3.0, 4.0, 5.0, and 10.0 ng/ml) of synthetic iPF2α-VI to aliquots of normal urine. The average of three samples at each concentration was used. Within-day (n = 5 or 6) and between-day (n = 6) reproducibility studies were performed at three different concentrations (1.0, 3.0, and 5.0 ng/ml) of synthetic iPF2α-VI standard. The recovery of the SPE procedure was evaluated at concentrations of 1.0, 5.0, and 10.0 ng/ml by adding [2H4]iPF2α-VI after extraction.
Clinical Studies.
Urinary iPs were characterized in 12 subjects (aged 4–43; six males and six females) with homozygous familial hypercholesterolemia (HFH). This condition was defined by the presence of childhood cutaneous or tendonous xanthomata, fasting total cholesterol levels of >500 mg/dl and normal triglyceride levels (<200 mg/dl). No subjects were cigarette smokers. None were diabetic or hypertensive and most were Caucasian (two African-American and one Asian). All subjects had a history and physical examination performed and were admitted to the General Clinical Research Center for a 48-hour period. Overnight 12-hour urine samples were collected before any blood draws or invasive testing was performed. Subsequently, fasting blood samples were taken for the assessment of lipoproteins and coronary angiography was performed to assess the presence of coronary disease. An age- and gender-matched sample of subjects (aged 5–43; six males and six females) was used as a control group (n = 12). They underwent similar overnight 12-hour urine sample collection and fasting lipoprotein assessment. We have previously described increased urinary iPF2α-III and iPF2α-VI in patients with HFH (27), using GC/MS assays. We wished to confirm and extend these studies by applying the HPLC/ESI/MS/MS technique to this population. New patients with hypercholesterolemia and age and gender matched controls were recruited to the General Clinical Research Center of the Hospital of the University of Pennsylvania and the Childrens’ Hospital of Philadelphia. Patients with cardiac failure were enrolled at the time of their initial inpatient assessment at the General Clinical Research Center of the Hospital of the University of Pennsylvania. Overnight 12-hour urine samples were collected before patients’ undergoing echocardiography, exercise testing, and cardiac catherization. Urine samples were collected in polyethylene bottles containing butylated hydroxyanisole. Samples were kept refrigerated during the collection period, after which they were immediately aliquoted and stored at −70°C until analysis. Under these conditions, urine can be stored without any significant change in F2-iP levels (13, 34).
Twenty-nine patients with congestive heart failure (CHF) were enrolled (age 52.5 ± 12.2 mean ± SD, six women, three blacks, eight with hypertension, six with diabetes, and seven cigarette smokers) in the second study. The etiology of their disease was ischemic in 15 (52%) and nonischemic in 14 (48); 11 (38%) had dilated cardiomyopathy and 3 (10%) had congenital heart disease. Seven (24%) had class II symptoms, twelve (41%) had class III symptoms, ten (35%) had class IV symptoms, and the mean ejection fraction was 20.3 ± 5% (mean ± SD). Total serum cholesterol levels (181 ± 58 vs. to 187 ± 69 mg/dl, P not significant) were similar in patients with ischemic cardiomyopathy (one woman, six hypertensives, three diabetic, and three cigarette smokers) compared with patients with nonischemic cardiomyopathy (five women, two hypertensives, three diabetics, and four cigarette smokers). Overnight 12-hour urine samples were collected from asymptomatic, age- and sex-matched control subjects (n = 29, age 48.7 ± 22.5, six women, three blacks, eight hypertensives, three cigarette smokers). All studies were approved by the respective institutional review boards and the General Clinical Research Center advisory committees.
Primary response variables of interest for this investigation were the individual iP isomers. For each response variable, a Student’s t test for independent samples was applied. Statistical tests for homogeneity of variance was performed to confirm model assumptions, and the data were considered to be inconsistent with the assumed model when test probability was less than the type I error rate of 20%. If so, then the nonparametric analogue to Student’s t test, the Mann–Whitney Wilcoxon rank sum test, was performed. All tests of hypothesis for CHF and HFH classification are two-sided, with statistical significance associated with a type I error rate of 5% or less.
Results
Selection of Class Specific Product Ions.
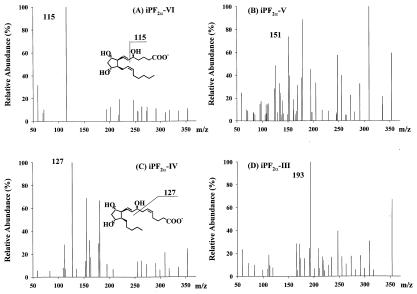
Isoprostane molecules readily generate abundant molecular ions (m/z 353) under ESI conditions in the negative-ion mode. The negatively charged molecular ions of the four classes of iPs (Fig. 1) undergo extensive collision-induced fragmentation (CID) at a collision energy of 23 eV. The product ion spectra of one selected synthetic standard from each of the four classes are illustrated (Fig. 2). The four isoprostanes generate very similar CID fragmentation patterns above m/z 200. However, they showed drastic differences below m/z 200. An ion at m/z 115 dominated the product ion spectrum of iPF2α-VI. Similarly and to a slightly lesser degree, the ion at m/z 193 is the most abundant ion in the iPF2α-III product ion spectrum. These two ions are also very class specific. The fragment ion at m/z 127 has the highest intensity for iPF2α-IV and is also very unique for this class of isomers. The choice for iPF2α-V was made based on the specificity of the product ion at m/z 151 (75% of the base peak intensity), even though it is not the base peak in the spectrum.
Figure 1.
Structures of the four classes of F2-isoprostanes formed from free radical (FR) reactions of arachidonic acid. Stereospecific structures of iPF2α-III and iPF2α-VI are shown, along with generic structures for class IV and class V.
Figure 2.
Product ion spectra of the four classes of isoprostanes. (A) iPF2α-VI. (B) iPF2α-V. (C) iPF2α-IV. (D) iPF2α-III.
The product ion structures and fragmentation pathways were studied by acquiring additional product ion spectra on the hydrogen–deuterium exchanged molecular species and those available deuterium labeled or 18O2 labeled molecular species (data not shown). For iPF2α-VI, the product ion spectrum of the hydrogen–deuterium-exchanged molecules still shows a very strong peak at m/z 115. So does 17,17,18,18-d4-iPF2α-VI. This suggests that the ion at m/z 115 is the charge-remote vinylic fragment of the molecular ion (35–38). Its formation includes a process of proton transfer from the hydroxyl group at C5 (Fig. 2). The ion at m/z 127 corresponds to an ion at m/z 128 in the deuterium-exchanged iPF2α-IV molecules. This suggests that this ion is formed through charge-remote allylic fragmentation (37, 38). The proton of the hydroxyl group at C8 is transferred to C5 (Fig. 2). The spectrum of the deuterium-exchanged molecular ions of iPF2α-V shows equal intensity peaks at m/z 151, 152, and 153. This leads to a conclusion of multiple competing pathways of ion formation and possibly involves different mechanisms of ring fragmentation. Similarly, iPF2α-III also generates three peaks of equal intensity at m/z 193, 194, and 195 after deuterium exchange. Multiple, competing pathways involving the 5-membered ring also appear to be relevant to this iP class. The fragment ion at m/z 193 also contains the lower numbered carbons because the 18O2-labeled molecular species give a fragment ion at m/z 197.
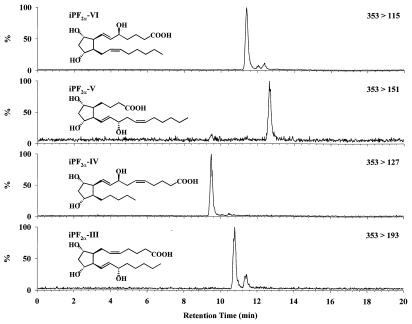
The specificity of the precursor to product ions of the four classes of iPs was verified by subjecting a mixture of one synthetic iP standard from each of the four classes to multiple reaction monitoring with suitable HPLC conditions. Good mass spectrometric resolution was achieved, illustrating the high specificity of each of the product ions (Fig. 3). Classes IV, V, and VI experience no interference from other classes. The detection of class III isoprostanes is complicated by class VI isomers. The class VI isomers give close to 10% of the ion signal of an equimolar amount of class III molecules. However, although class VI interferes with class III, interference does not occur in the other direction. Thus, efficient HPLC separation may be used to solve any ambiguities.
Figure 3.
HPLC/MS/MS of a mixture of four synthetic isoprostanes. Representatives of each of the four F2-iP classes were mixed and analyzed during a single multiple reaction-monitoring LC/MS/MS run. Four product ions were monitored (see Fig. 2), demonstrating the high degree of specificity of the selected ions.
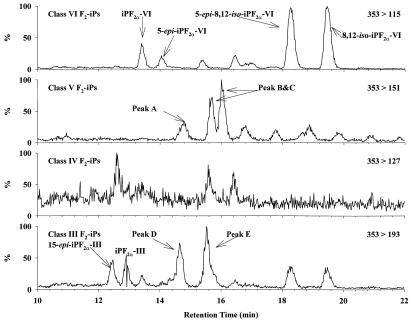
When urine samples from healthy volunteers are subjected to these HPLC/MS/MS conditions, there are eight identifiable peaks of class VI isomers, corresponding nicely to the eight pairs of enantiomers predicted theoretically (Fig. 4; refs. 19–21). There are also a few relatively large peaks corresponding to class III and V isomers whereas only trace amounts of class IV isomers can be detected. The class identities of some of the larger peaks have been verified by their product ion spectra. The structure of several of the peaks have also been identified by subsequent studies comparing their HPLC and/or GC/MS characteristics to synthetic standards (Fig. 4; ref. 12).
Figure 4.
HPLC/MS/MS of urinary F2-isoprostanes. Selected peaks were identified by comparison with synthetic standards. Other quantified peaks are labeled alphabetically in the chromatograms. Among these unidentified compounds, iPF2α-V peaks B and C were quantified together as one peak because of insufficient chromatographic resolution.
Characteristics of the Assay.
Sample preparation utilized a single solid phase extraction (SPE) using Varian BondElut LMS polymer 100 mg RP-SPE cartridges. The average recoveries obtained using the LMS polymer phase cartridges were 97, 112, and 88% for iPF2α-VI concentrations of 1.0, 5.0, and 10.0 ng/ml, respectively.
We selected iPF2α-VI as a model compound. The limit of detection was 5 pg injected on column requiring a signal-to-noise ratio of ≥3. The assay is linear over the range of 10 pg to 50 ng injected on column. A 2.0-mm HPLC column requires a relatively high flow rate of 200 μl/min to achieve optimal separation of the isomers in urine samples. A 4:1 postcolumn split was then introduced to reduce the flow rate of the HPLC effluent to 50 μl/min, which is the appropriate flow to the source.
A potential limitation of ESI/MS/MS is an adverse effect of the sample matrix on the signal intensity of analyte ions (39). To account for such potential effects, the linearity of the assay was also verified by adding iPF2α-VI synthetic standards to authentic urine samples. Three sets of samples were prepared with six different concentrations of added iP ranging between 0 and 10 ng/ml. Plotting the measured iPF2α-VI concentrations vs. amount of standard added provides a linear relationship described by the equation y = 1.06x + 2.15. The correlation coefficient (r) for the two variables is 0.99. The results of the within- and between-day precision estimates of the assay are presented in Table 1.
Table 1.
Assay reproducibility for iPF2α-VI in human urine (n = 6)
| Sample Conc., ng/ml | Within-day precision
|
Between-day precision
|
||||
|---|---|---|---|---|---|---|
| Mean† | SD | %CV* | Mean† | SD | %CV* | |
| 1 | 0.320‡ | 0.008 | 0.026 | 0.323 | 0.017 | 0.054 |
| 3 | 0.538 | 0.013 | 0.025 | 0.532 | 0.021 | 0.039 |
| 5 | 0.741 | 0.041 | 0.056 | 0.749 | 0.017 | 0.023 |
*Coefficient of variation
†Peak area ratios of d0/d4 iPF2α-VI.
‡n = 5.
Clinical Studies.
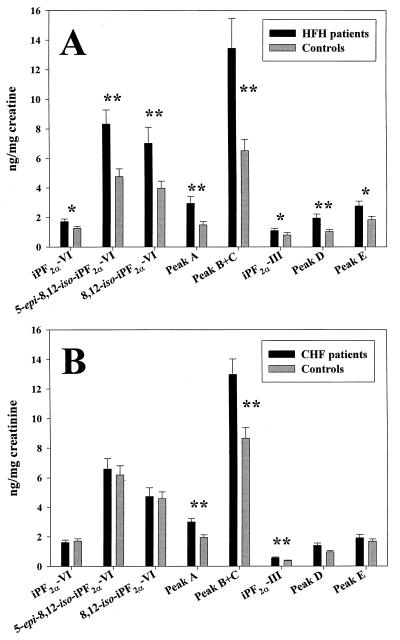
Urinary levels of all classes of F2-iPs were increased in HFH patients compared with controls (Fig. 5A). There was a marked heterogeneity in the excretion of individual isomers ranging from 1.11 ± 0.45 pg/mg creatinine for iPF2α-III to 8.33 ± 3.17 pg/mg creatinine for 5-epi-8,12-iso-iPF2α-VI. However, the ratio of increased excretion of individual isomers in HFH patients compared with control subjects remained remarkably constant. These findings correspond closely to our recent report of significant increases in urinary iPF2α-III (mean 1.5-fold) and iPF2α-VI (mean 1.6-fold) in a larger group of HFH patients compared with matched control subjects (27) using previously described GC/MS assays (10, 11).
Figure 5.
F2-iP levels in HFH and CHF. (A) HFH patients (n = 12) vs. controls (n = 12). (B) CHF patients (n = < 29) vs. controls (n = < 29). For identification of the peaks, see Fig. 4. *, P < 0.05; **, P < 0.01.
Paired analysis revealed that all individual isomers measured were significantly elevated in the HFH group (Fig. 5A). The increase in mean levels varied from 1.36-fold for iPF2α-VI (P < 0.05) to 2.06-fold for iPF2α-V peaks B and C combined (P < 0.01). The most abundant isomer characterized in the present studies was 5-epi-8,12-iso-iPF2α-VI, which was 1.75-fold higher (P < 0.05) in the patients with HFH than in controls. The increments in iPF2α-III and iPF2α-VI were 1.37-fold (P < 0.05) and 1.36-fold (P < 0.05) in the present study, corresponding well with the increments previously measured by GC/MS.
Paired analysis of iPF2α-VI in HFH patients and controls by LC/MS/MS and GC/MS gave similar results (1.49 ± 0.56 ng/mg creatinine vs. 1.54 ± 0.60 ng/mg creatinine, respectively), which were highly correlated (r = 0.93; P < 0.0001; n = 24). We have recently established a GC/MS assay for 8,12-iso iPF2α-VI (12). The paired analysis of this iP in CHF patients or controls by GC/MS and LC/MS/MS assay also showed a high degree of correlation (r = 0.92; P < 0.0001; n = 58).
The pattern in CHF was much less striking (Fig. 5B). The mean ratios of patients vs. controls were close to unity (0.94, 1.07, and 1.03) for the three class VI isomers measured here, iPF2α-VI, 5-epi-8,12-iso-iPF2α-VI and 8,12-iso-iPF2α-VI respectively. The highest ratio was 1.55 for iPF2α-V peak A. The most abundant isomer characterized in this study was 5-epi-8,12-iso-iPF2α-VI, which was 6.58 ± 3.77 ng/mg creatinine in the patients and 6.18 ± 3.23 in the controls (P not significant). Paired analysis by Student’s t test identified significant differences (P < 0.005) between the groups for peak B + C, peak A, and iPF2α-III. There was no difference in the urinary levels of any isomer between patients with ischemic (n = 15) vs. nonischemic (n = 14) cardiomyopathy. For example, the corresponding values for iPF2α-VI were 6.25 ± 3.7 ng/mg creatine vs. 6.94 ± 3.9 ng/mg creatine and for iPF2α 0.56 ± 0.08 ng/mg creatinine vs. 0.56 ± 0.07 ng/mg creatine.
Discussion
Isoprostanes are a complex mixture of isomeric prostaglandin species formed in a free radical-dependent manner from arachidonic acid. Because of their chemical stability and their potential detection at their site of formation (34, 40), as well as in plasma and in urine (22, 27, 28, 41), they have particular attraction as indices of oxidant stress in vivo. Such measurements might usefully provide a biochemical rationale for the selection of diseases and dosage requirements when designing clinical trials of antioxidants. Most attention on isoeicosanoid analysis has focused on the F2-iPs, although interest has broadened recently to encompass other species (42, 43). Although a GC/MS assay for iPs using [2H4]PGF2α as an internal standard has been reported (41), a limitation has been the lack of homologous standards corresponding to the unidentified components of the heterogeneous peak selected for measurement (2). We have developed a series of assays for individual iPs, using GC/MS and homologous internal standards. This approach has yielded much information in support of the usefulness of iP analysis as an index of oxidant stress in vivo. For example, suppression of elevated iPF2α-VI generation with vitamin E retards the development of experimental atherosclerosis in mice, despite continued hypercholesterolemia (18), providing support for the functional importance of lipid peroxidation in this condition (44). We have also demonstrated that hypercholesterolemic patients, like the mice, have increased urinary concentrations of this iP (27), suggesting that a similar interventional strategy might be applicable to clinical trials.
Despite these observations, there are potential limitations to an approach based on analysis of a single iP. For example, the dominant iP formed in the tissue of origin might not be the most abundant iP in urine. The most commonly used GC/MS assays for iPs utilize the highly sensitive, negative ion chemical ionization mode. Although LC/MS/MS retains the specificity of negative ion chemical ionization GC/MS, it is generally a less sensitive technique. However, much of the sample preparation and attendant loss of recovery associated with GC/MS is omitted before LC/MS/MS analysis. For example, negative ion chemical ionization analysis of iPF2α-III and iPF2α-VI by GC/MS requires at least one SPE step, at least one TLC step, two derivatization steps, and extracting and/or drying the sample between each of these steps. By contrast, one SPE, one drying step, and a centrifugal filtration are all that are required before analysis by LC/MS/MS. As we report, the assay is highly sensitive and is reproducible when performed in human urine. Indeed, in the present study, the values of 8,12-iso-iPF2α-VI, an abundant F2-iP in human urine (12), analyzed by negative ion chemical ionization GC/MS and by LC/MS/MS in the urine of patients with CHF and their controls were highly correlated. The additional information afforded by the latter technique and its ready adaptability to high throughput analyses would favor its application in many studies of iP generation.
Although a focus on a single, less-abundant iP might fail to detect alterations in its generation because of insufficient assay sensitivity, the assumption that quantitative changes in iP generation under conditions of oxidant stress would be reflected similarly by all detectable isomers of a given eicosanoid also remains to be tested. Such information would be pertinent to the selection of target analytes for immunoassay development, as well as to the interpretation of information based on analysis of single iPs. Immunoassays for iPF2α-III are available commercially, but this is a relatively minor F2-iP in urine (12). Such assays have not been checked for cross reactivity with most other F2-iPs or any of their potential metabolites. An issue that seems particular to this iP is its capacity for formation by either COX-1 or COX-2 (10, 22). Although these enzymatic pathways do not seem to contribute detectably to urinary iPF2α-III in studies to date (26), this remains a caveat to the interpretation of investigations based on analysis of this iP alone.
We have included the specific detection of selected members of three of the four structural classes of the F2-iPs in the present assay and intend to characterize the identity of the compounds corresponding to the remaining peaks that are abundant in human urine. Murphy and coworkers have used LC/MS/MS to analyze iPs in the livers of choloroform-treated rats (45). Although multiple internal standards were not available, the most abundant F2-isomers that they observed in the livers of chloroform-treated rats were of class VI (45), which is in good accordance with the predominance of 5-epi-8,12-iso-iPF2α-VI and 8,12-iso-iPF2α-VI in human urine. Using this approach, we find that a range of urinary F2-iPs are elevated to a roughly comparable degree in patients with HFH. This extends our previous observations with GC/MS analysis of two discrete iPs, iPF2α-III and iPF2α-VI. Again, in the case of these iPs, analysis by LC/MS/MS appears consistent with the fold increase in HFH. By contrast, we find no evidence of a similar broad increase in urinary iPs in the patients with severe congestive heart failure. However, pairwise comparisons suggest increases in some of the individual iPs. Although there have been suggestions of elevated oxidant stress in such patients (46–48), this has been based on potentially fallible indices of lipid peroxidation (49). Previously, Mallat and colleagues reported that immunoreactive iPF2α-III was elevated in the pericardial fluid of such patients (50). Future studies should determine whether the disparate results among urinary iPs in the present study reflect the play of chance or differences in the generation, metabolism, or clearance of individual iPs in CHF.
In summary, we report the development of an LC/MS/MS assay for distinct members of the structural classes of the F2-iPs in human urine. It is highly reproducible, sensitive, and specific and is readily adaptable to high throughput analyses in complex biological matrices. Application of this assay to clinical investigation demonstrates that a broad range of F2-iPs are increased in the urine of patients with HFH, suggesting coordinate generation of these compounds in a syndrome of oxidant stress. By contrast, evidence for increased generation of only some of the measured F2-iPs was observed in congestive heart failure. This approach should be useful in addressing fundamental questions relating to iP formation, disposition, and excretion in humans.
Acknowledgments
This work was supported by Grants HL-62250, M01RR0040, and DK-44730 from the National Institutes of Health and an AMX-360 NMR Instrument (Grant CHE-9013145) from the National Science Foundation. Dr. FitzGerald is the Robinette Foundation Professor of Cardiovascular Medicine.
Abbreviations
- iP
isoprostane
- COX
cyclooxygenase
- ESI
electrospray ionization
- SPE
solid phase extraction
- HFH
hypercholesterolemia
- CHF
congestive heart failure
References
- 1.Morrow J D, Roberts L J, II. Biochem Pharmacol. 1996;51:1–9. doi: 10.1016/0006-2952(95)02072-1. [DOI] [PubMed] [Google Scholar]
- 2.Lawson J A, Rokach J, FitzGerald G A. J Biol Chem. 1999;274:24441–24444. doi: 10.1074/jbc.274.35.24441. [DOI] [PubMed] [Google Scholar]
- 3.Morrow J D, Awad J A, Boss H J, Blair I A, Roberts L J, II. Proc Natl Acad Sci USA. 1992;89:10721–10725. doi: 10.1073/pnas.89.22.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi K, Nammour T M, Fukunaga M, Ebert J, Morrow J D, Roberts L J, II, Hoover R I, Badr K F. J Clin Invest. 1992;90:1276–1280. doi: 10.1172/JCI115826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kingsley Y, Halushka P V, Yan Y-T, Wong P Y-K. J Pharmacol Exp Ther. 1994;270:1192–1996. [PubMed] [Google Scholar]
- 6.Elmhurst J L, Betti P-A, Rangachari P K. J Pharmacol Exp Ther. 1997;283:1198–1205. [PubMed] [Google Scholar]
- 7.Pratico D, Smyth E, Violi F, FitzGerald G A. J Biol Chem. 1996;271:14916–14924. doi: 10.1074/jbc.271.25.14916. [DOI] [PubMed] [Google Scholar]
- 8.Kunapuli P, Lawson J A, Rokach J A, Meinkoth J L, FitzGerald G A. J Biol Chem. 1998;273:22442–22452. doi: 10.1074/jbc.273.35.22442. [DOI] [PubMed] [Google Scholar]
- 9.McNamara P, Lawson J A, Rokach J, FitzGerald G A. FASEB J. 1999;13:A1549. [Google Scholar]
- 10.Pratico D, Lawson J A, FitzGerald G A. J Biol Chem. 1995;270:9800–9808. doi: 10.1074/jbc.270.17.9800. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, Ciabattoni G, Creminon C, Lawson J A, FitzGerald G A, Patrono C, Maclouf J. J Pharmacol Exp Ther. 1995;275:94–100. [PubMed] [Google Scholar]
- 12.Lawson J, Rokach J, Hongwei L, Adiyaman M, Hwang S-W, Khanapure S P, FitzGerald G A. J Biol Chem. 1998;273:29295–29301. doi: 10.1074/jbc.273.45.29295. [DOI] [PubMed] [Google Scholar]
- 13.Praticò D, Barry O P, Lawson J A, Adiyaman M, Hwang S-W, Khanapure S P, Iuliano L, Rokach J, FitzGerald G A. Proc Natl Acad Sci USA. 1998;95:3449–3454. doi: 10.1073/pnas.95.7.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiabrando C, Valagussa A, Rivalta C, Durand T, Guy A, Zuccato E, Villa P, Rossi J-C, Fanelli R. J Biol Chem. 1999;274:1313–1319. doi: 10.1074/jbc.274.3.1313. [DOI] [PubMed] [Google Scholar]
- 15.Davi G, Ciabattoni G, Consoli A, Mezzetti A, Falco A, Santarone S, Pennese E, Vitacolonna E, Bucciarelli T, Costantini F, et al. Circulation. 1999;99:224–229. doi: 10.1161/01.cir.99.2.224. [DOI] [PubMed] [Google Scholar]
- 16.Tsikas D, Schwedhelm E, Fauler J, Gutzki F M, Mayatepek E, Frolich J C. J Chromatogr B Biomed Sci Appl. 1998;716:7–17. doi: 10.1016/s0378-4347(98)00275-8. [DOI] [PubMed] [Google Scholar]
- 17.Leo M A, Aleynik S I, Siegel J H, Kasmin F E, Aleynik M K, Lieber C S. Am J Gastroenterol. 1997;92:2069–2072. [PubMed] [Google Scholar]
- 18.Pratico D, Tangirala R K, Rader D J, Rokach J, FitzGerald G A. Nat Med. 1998;4:1189–1192. doi: 10.1038/2685. [DOI] [PubMed] [Google Scholar]
- 19.Awad J A, Morrow J D, Takahashi K, Roberts L J, II. J Biol Chem. 1993;268:4161–4169. [PubMed] [Google Scholar]
- 20.Waugh R J, Murphy R C. J Am Soc Mass Spectrom. 1995;7:490–499. doi: 10.1016/1044-0305(95)00709-1. [DOI] [PubMed] [Google Scholar]
- 21.Rokach J, Khanapure S P, Hwang S-W, Adiyaman M, Schio L, FitzGerald G A. Synthesis. 1998;1:569–580. [Google Scholar]
- 22.Pratico D, FitzGerald G A. J Biol Chem. 1996;271:8919–8924. doi: 10.1074/jbc.271.15.8919. [DOI] [PubMed] [Google Scholar]
- 23.Kerins D M, Shuh M, Kunitada S, FitzGerald G A, Fitzgerald D J. J Pharmacol Exp Ther. 1991;257:487–492. [PubMed] [Google Scholar]
- 24.Delanty N, Reilly M, Lawson J A, McCarthy J, Wood F, Fitzgerald D J, FitzGerald G A. Circulation. 1997;95:2492–2499. doi: 10.1161/01.cir.95.11.2492. [DOI] [PubMed] [Google Scholar]
- 25.Nowak J, Murray J J, Oates J A, FitzGerald G A. Circulation. 1987;76:6–14. doi: 10.1161/01.cir.76.1.6. [DOI] [PubMed] [Google Scholar]
- 26.Reilly M, Delanty N, Lawson J A, FitzGerald G A. Circulation. 1996;94:19–25. doi: 10.1161/01.cir.94.1.19. [DOI] [PubMed] [Google Scholar]
- 27.Reilly M, Praticó D, Delanty N, DiMinno G, Tremoli E, Rader D, Kapoor S, Rokach J, Lawson J, FitzGerald G A. Circulation. 1998;98:2822–2828. doi: 10.1161/01.cir.98.25.2822. [DOI] [PubMed] [Google Scholar]
- 28.Pratico D, Trojanowski J, Lee V, Rokach J, FitzGerald G A. FASEB J. 1998;12:1777–1783. doi: 10.1096/fasebj.12.15.1777. [DOI] [PubMed] [Google Scholar]
- 29.Praticó D, Juliano J, Mauriello A, Spagnoli S, Lawson J, Rokach J, Maclouf J, Violi F, FitzGerald G A. J Clin Invest. 1997;100:2028–2034. doi: 10.1172/JCI119735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adiyaman M, Lawson J A, Hwang S, Khanapure S P, FitzGerald G A, Rokach J. Tetrahedron Lett. 1996;37:4849–4852. [Google Scholar]
- 31.Adiyaman M, Li H, Lawson J A, Hwang S, Khanapure S P, FitzGerald G A, Rokach J. Tetrahedron Lett. 1997;38:3339–3342. [Google Scholar]
- 32.Hwang S W, Adiyaman M, Lawson J A, FitzGerald G A, Rokach J. Tetrahedron Lett. 1999;40:6167–6171. [Google Scholar]
- 33.Westcott J Y, Clay K L, Murphy R C. Biomed Mass Spectrom. 1985;12:714. doi: 10.1002/bms.1200121208. [DOI] [PubMed] [Google Scholar]
- 34.Morrow J D, Hill K E, Burk R F, Nammour T M, Badr K F, Roberts L J, II. Proc Natl Acad Sci USA. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Contado M J, Adams J, Gross M L. Adv Mass Spectrom. 1989;11B:1034–1035. [Google Scholar]
- 36.Zirrilli J A, Davoli E, Bettazzoli L, Gross M, Murphy R C. J Am Soc Mass Spectrom. 1990;1:325–335. doi: 10.1016/1044-0305(90)85009-B. [DOI] [PubMed] [Google Scholar]
- 37.Wheelan P, Zirrolli J A, Murphy R C. Biol Mass Spectrom. 1993;22:465–473. doi: 10.1002/bms.1200220808. [DOI] [PubMed] [Google Scholar]
- 38.Wheelan P, Zirrolli J A, Murphy R C. J Am Soc Mass Spectrom. 1996;7:140–149. doi: 10.1016/1044-0305(95)00628-1. [DOI] [PubMed] [Google Scholar]
- 39.Buhrman D L, Price P I, Rudewicz P J. J Am Soc Mass Spectrom. 1996;7:1099–1105. doi: 10.1016/S1044-0305(96)00072-4. [DOI] [PubMed] [Google Scholar]
- 40.Patrono C, FitzGerald G A. Arterioscler Throm Vasc Biol. 1997;17:2309–2315. doi: 10.1161/01.atv.17.11.2309. [DOI] [PubMed] [Google Scholar]
- 41.Morrow J D, Roberts L J, II. Methods Enzymol. 1994;233:163–174. doi: 10.1016/s0076-6879(94)33019-0. [DOI] [PubMed] [Google Scholar]
- 42.Mallat Z, Nakamura T, Ohan J, Leseche G, Tedgui A, Maclouf J, Murphy R C. J Clin Invest. 1999;103:421–427. doi: 10.1172/JCI3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y, Morrow J D, Jackson L J, II. J Biol Chem. 1999;274:10863–10868. doi: 10.1074/jbc.274.16.10863. [DOI] [PubMed] [Google Scholar]
- 44.Witztum J L. Circulation. 1998;25:2785–2787. doi: 10.1161/01.cir.98.25.2785. [DOI] [PubMed] [Google Scholar]
- 45.Waugh R J, Morrow J D, Roberts L J, II, Murphy R C. Free Radical Biol Med. 1997;23:943–954. doi: 10.1016/s0891-5849(97)00133-0. [DOI] [PubMed] [Google Scholar]
- 46.Kukin M L, Kalman J, Charney R H, Levy D K, Buchholz-Varley C, Ocampo O N, Eng E. Circulation. 1999;31:1352–1356. doi: 10.1161/01.cir.99.20.2645. [DOI] [PubMed] [Google Scholar]
- 47.Keith M, Geranmayegan A, Sole M J, Kurlan R, Robinson A, Omran A S, Jeejeebhoy K N. J Am Coll Cardiol. 1998;31:1352–1356. doi: 10.1016/s0735-1097(98)00101-6. [DOI] [PubMed] [Google Scholar]
- 48.Nishiyama Y, Ikeda H, Haramaki N, Yoshida N, Imaizumi T. Am Heart J. 1998;135:115–120. doi: 10.1016/s0002-8703(98)70351-5. [DOI] [PubMed] [Google Scholar]
- 49.Liu J K, Yeo H C, Doniger S J, Ames B N. Anal Biochem. 1997;245:161–166. doi: 10.1006/abio.1996.9990. [DOI] [PubMed] [Google Scholar]
- 50.Mallat Z, Philip I, Lebret M, Chatel D, Maclouf J, Tedgui A. Circulation. 1998;97:1536–1539. doi: 10.1161/01.cir.97.16.1536. [DOI] [PubMed] [Google Scholar]