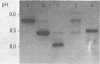
Abstract
A new chromatographic procedure was developed which obtained highly purified preparations of staphylococcal enterotoxins B and C1 in yields of 60% from cultures of Staphylococcus aureus and which is faster than any of the separation methods used previously. The procedure involves chromatography on carboxymethylcellulose, removal of alpha-toxin by adsorption to rabbit erythrocyte membranes, and finally, chromatofocusing as the fundamental new step. Enterotoxins were obtained in highly purified form and behaved in a homogeneous manner as determined by ultracentrifugation and electrophoresis on polyacrylamide gel in the presence of sodium dodecyl sulfate, with molecular weights of 34,000 for staphylococcal enterotoxin B and 30,000 for staphylococcal enterotoxin C1. Using chromatofocusing as the final purification step, we isolated three B and six C1 distinct but immunologically identical enterotoxin fractions, which were found to be devoid of any impurities and to possess a marked degree of toxicity in monkeys.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergdoll M. S., Borja C. R., Avena R. M. Identification of a new enterotoxin as enterotoxin C. J Bacteriol. 1965 Nov;90(5):1481–1485. doi: 10.1128/jb.90.5.1481-1485.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergdoll M. S., Huang I. Y., Schantz E. J. Chemistry of the staphylococcal enterotoxins. J Agric Food Chem. 1974 Jan-Feb;22(1):9–13. doi: 10.1021/jf60191a001. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Füssle R., Tranum-Jensen J. Staphylococcal alpha-toxin: oligomerization of hydrophilic monomers to form amphiphilic hexamers induced through contact with deoxycholate detergent micelles. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5475–5479. doi: 10.1073/pnas.78.9.5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borja C. R., Bergdoll M. S. Purification and partial characterization of enterotoxin C produced by Staphylococcus aureus strain 137. Biochemistry. 1967 May;6(5):1467–1473. doi: 10.1021/bi00857a032. [DOI] [PubMed] [Google Scholar]
- CASMAN E. P., BERGDOLL M. S., ROBINSON J. DESIGNATION OF STAPHYLOCOCCAL EXTEROTOXINS. J Bacteriol. 1963 Mar;85:715–716. doi: 10.1128/jb.85.3.715-716.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P. C., Dickie N. Fractionation of staphylococcal enterotoxin B by isoelectric focusing. Biochim Biophys Acta. 1971 May 25;236(2):367–375. doi: 10.1016/0005-2795(71)90217-0. [DOI] [PubMed] [Google Scholar]
- Chang P. C., Dickie N. Heterogeneity of staphylococcal enterotoxin B. Can J Microbiol. 1971 Nov;17(11):1479–1481. doi: 10.1139/m71-237. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Dalen A. B. Multiple forms of staphylococcal alpha-toxin. Acta Pathol Microbiol Scand Suppl. 1975 Dec;83(6):561–568. doi: 10.1111/j.1699-0463.1975.tb00139.x. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell M. R., Liu C. T., Spertzel R. O., Beisel W. R. Mechanisms of oral staphylococcal enterotoxin B-induced emesis in the monkey (38553). Proc Soc Exp Biol Med. 1975 Feb;148(2):424–427. doi: 10.3181/00379727-148-38553. [DOI] [PubMed] [Google Scholar]
- Freer J. H., Arbuthnott J. P., Bernheimer A. W. Interaction of staphylococcal alpha-toxin with artificial and natural membranes. J Bacteriol. 1968 Mar;95(3):1153–1168. doi: 10.1128/jb.95.3.1153-1168.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT G. A., GOUREVITCH A., LEIN J. Preservation of cultures by drying on porcelain beads. J Bacteriol. 1958 Oct;76(4):453–454. doi: 10.1128/jb.76.4.453-454.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang I. Y., Bergdoll M. S. The primary structure of staphylococcal enterotoxin B. 3. The cyanogen bromide peptides of reduced and aminoethylated enterotoxin B, and the complete amino acid sequence. J Biol Chem. 1970 Jul 25;245(14):3518–3525. [PubMed] [Google Scholar]
- Kekwick R. A. The serum proteins in multiple myelomatosis. Biochem J. 1940 Sep;34(8-9):1248–1257. doi: 10.1042/bj0341248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kickhöfen B., Warth R. A rapid method for dry weight determination of proteins on a micro scale with an electrobalance. Hoppe Seylers Z Physiol Chem. 1976 May;357(5):745–749. doi: 10.1515/bchm2.1976.357.1.745. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Metzger J. F., Johnson A. D., Collins W. S., 2nd Fractionation and purification of Staphylococcus aureus enterotoxin B by electrofocusing. Biochim Biophys Acta. 1972 Jan 26;257(1):183–186. doi: 10.1016/0005-2795(72)90269-3. [DOI] [PubMed] [Google Scholar]
- Metzger J. F., Johnson A. D., Spero L. Intrinsic and chemically produced microheterogeneity of Staphylococcus aureus enterotoxin type C. Infect Immun. 1975 Jul;12(1):93–97. doi: 10.1128/iai.12.1.93-97.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEGEMANN H. Eine Mikrobestimmung von Amid-Stickstoff, speziell in Proteinen. Hoppe Seylers Z Physiol Chem. 1958;312(4-6):255–263. [PubMed] [Google Scholar]
- Schaeffer W. I., Gabliks J., Calitis R. Interaction of staphylococcal enterotoxin B with cell cultures of human embryonic intestine. J Bacteriol. 1966 Jan;91(1):21–26. doi: 10.1128/jb.91.1.21-26.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer W. I., Gabliks J., Calitis R. Interference by trypsin in the interaction of staphylococcal enterotoxin B and cell cultures of human embryonic intestine. J Bacteriol. 1967 May;93(5):1489–1492. doi: 10.1128/jb.93.5.1489-1492.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer W. I. Interaction of staphylococcal enterotoxin B with cell cultures I. Effect of serum and testing procedures. Infect Immun. 1970 May;1(5):455–458. doi: 10.1128/iai.1.5.455-458.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz E. J., Roessler W. G., Wagman J., Spero L., Dunnery D. A., Bergdoll M. S. Purification of staphylococcal enterotoxin B. Biochemistry. 1965 Jun;4(6):1011–1016. doi: 10.1021/bi00882a005. [DOI] [PubMed] [Google Scholar]
- Schmidt J. J., Spero L. The complete amino acid sequence of staphylococcal enterotoxin C1. J Biol Chem. 1983 May 25;258(10):6300–6306. [PubMed] [Google Scholar]
- Six H. R., Harshman S. Physical and chemical studies on staphylococcal -toxins A and B . Biochemistry. 1973 Jul 3;12(14):2677–2683. doi: 10.1021/bi00738a020. [DOI] [PubMed] [Google Scholar]
- Spencer R. L., Wold F. A new convenient method for estimation of total cystine-cysteine in proteins. Anal Biochem. 1969 Oct 15;32(1):185–190. doi: 10.1016/0003-2697(69)90123-7. [DOI] [PubMed] [Google Scholar]
- Spero L., Griffin B. Y., Middlebrook J. L., Metzger J. F. Effect of single and double peptide bond scission by trypsin on the structure and activity of staphylococcal enterotoxin C. J Biol Chem. 1976 Sep 25;251(18):5580–5588. [PubMed] [Google Scholar]
- Sugiyama H., Hayama T. Abdominal viscera as site of emetic action for staphylococcal enterotoxin in the monkey. J Infect Dis. 1965 Oct;115(4):330–336. doi: 10.1093/infdis/115.4.330. [DOI] [PubMed] [Google Scholar]
- Thelestam M., Möllby R. Determination of toxin-induced leakage of different-size nucleotides through the plasma membrane of human diploid fibroblasts. Infect Immun. 1975 Apr;11(4):640–648. doi: 10.1128/iai.11.4.640-648.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelestam M., Möllby R., Wadström T. Effects of staphylococcal alpha-, beta-, delta-, and gamma-hemolysins on human diploid fibroblasts and HeLa cells: evaluation of a new quantitative as say for measuring cell damage. Infect Immun. 1973 Dec;8(6):938–946. doi: 10.1128/iai.8.6.938-946.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagman J., Edwards R. C., Schantz E. J. Molecular size, homogeneity, and hydrodynamic properties of purified staphylococcal enterotoxin B. Biochemistry. 1965 Jun;4(6):1017–1023. doi: 10.1021/bi00882a006. [DOI] [PubMed] [Google Scholar]
- Warren J. R., Spero L., Metzger J. F. Isothermal denaturation of aqueous staphylococcal enterotoxin B by guanidine hydrochloride, urea, and acid pH. Biochemistry. 1974 Apr 9;13(8):1678–1683. doi: 10.1021/bi00705a019. [DOI] [PubMed] [Google Scholar]