Abstract
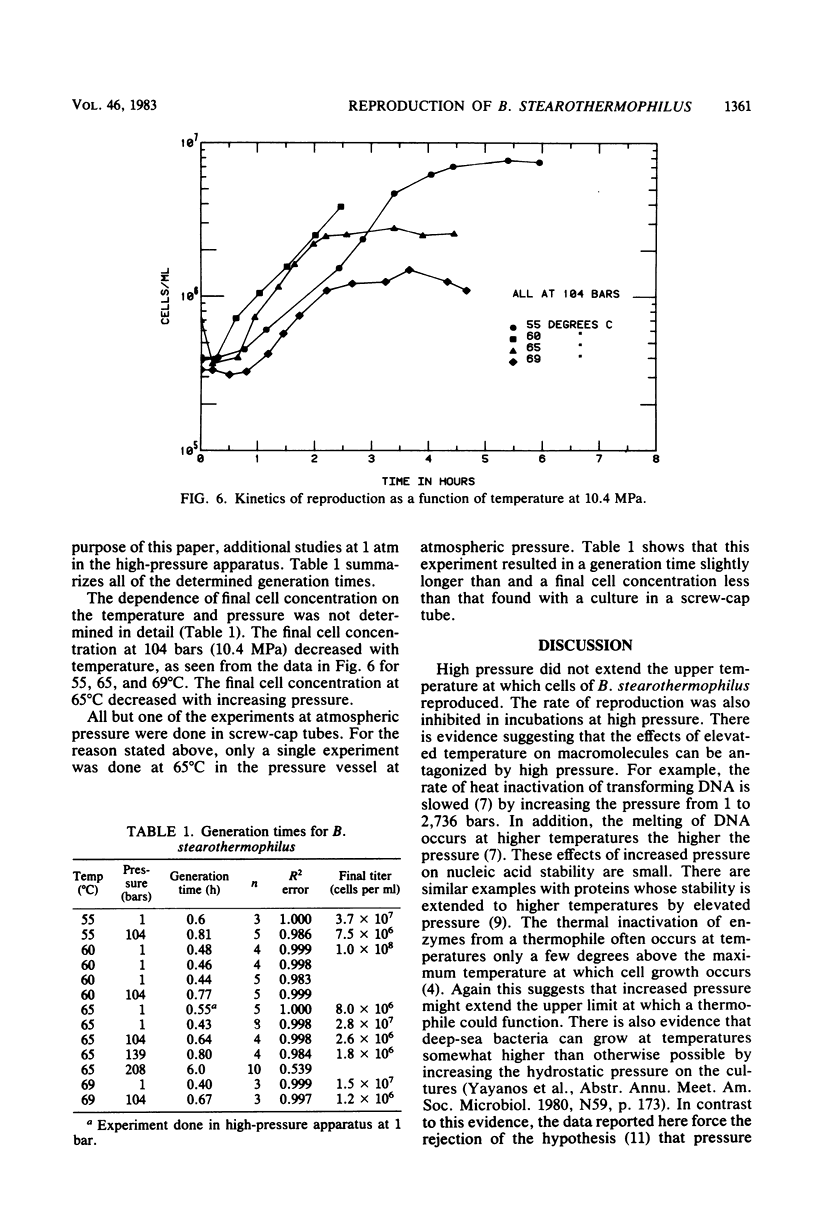
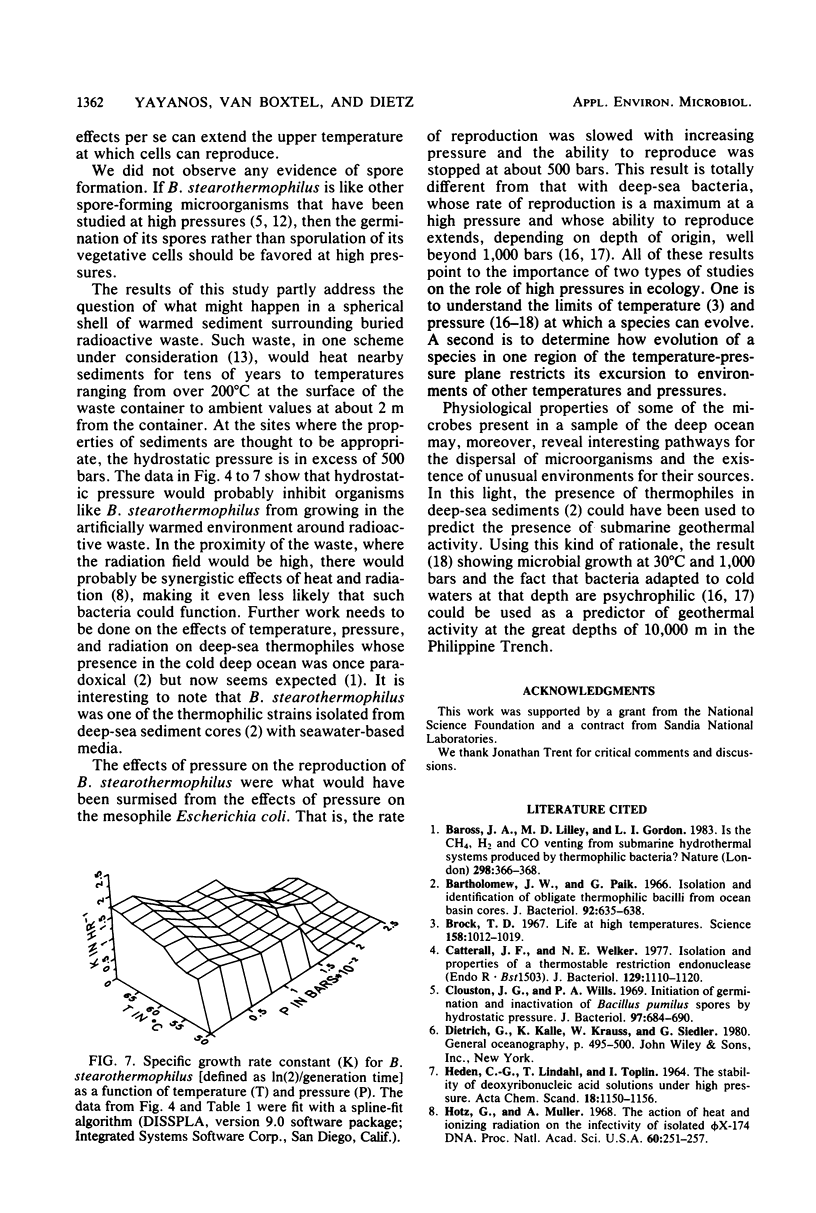
The colony-forming ability and the rate of reproduction of Bacillus stearothermophilus were determined as a function of temperature and pressure. Colonies were formed between 39 and 70°C at atmospheric pressure and between 54 and 67°C at 45 MPa. Colonies did not form at 55.9 MPa. The rate of reproduction in broth cultures decreased with increasing pressure at all temperatures. The rate of reproduction diminished rapidly with pressure above 10.4 MPa. Therefore, increased hydrostatic pressure was not sufficient to enable B. stearothermophilus to function beyond the temperature limiting growth and reproduction at atmospheric pressure, and B. stearothermophilus should grow in naturally or artificially warmed regions of the deep sea, where the pressure is less than approximately 50 MPa, although growth rates would be low above 10 MPa.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartholomew J. W., Paik G. Isolation and identification of obligate thermophilic sporeforming bacilli from ocean basin cores. J Bacteriol. 1966 Sep;92(3):635–638. doi: 10.1128/jb.92.3.635-638.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D. Life at high temperatures. Evolutionary, ecological, and biochemical significance of organisms living in hot springs is discussed. Science. 1967 Nov;158(3804):1012–1019. doi: 10.1126/science.158.3804.1012. [DOI] [PubMed] [Google Scholar]
- Catterall J. F., Welker N. E. Isolation and properties of a thermostable restriction endonuclease (ENDO R-Bst1503). J Bacteriol. 1977 Feb;129(2):1110–1120. doi: 10.1128/jb.129.2.1110-1120.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouston J. G., Wills P. A. Initiation of germination and inactivation of Bacillus pumilus spores by hydrostatic pressure. J Bacteriol. 1969 Feb;97(2):684–690. doi: 10.1128/jb.97.2.684-690.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotz G., Müller A. The action of heat and ionizing radiation on the infectivity of isolated phi-X-174 DNA. Proc Natl Acad Sci U S A. 1968 May;60(1):251–257. doi: 10.1073/pnas.60.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell W. G., Wills P. A. Initiation of Bacillus spore germination by hydrostatic pressure: effect of temperature. J Bacteriol. 1977 Mar;129(3):1272–1280. doi: 10.1128/jb.129.3.1272-1280.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayanos A. A. A technique for studying biological reaction rates at high pressure. Rev Sci Instrum. 1969 Jul;40(7):961–963. doi: 10.1063/1.1684124. [DOI] [PubMed] [Google Scholar]
- Yayanos A. A., Dietz A. S., Van Boxtel R. Dependence of reproduction rate on pressure as a hallmark of deep-sea bacteria. Appl Environ Microbiol. 1982 Dec;44(6):1356–1361. doi: 10.1128/aem.44.6.1356-1361.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayanos A. A., Dietz A. S., Van Boxtel R. Obligately barophilic bacterium from the Mariana trench. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5212–5215. doi: 10.1073/pnas.78.8.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobell C. E. Bacterial Life at the Bottom of the Philippine Trench. Science. 1952 May 9;115(2993):507–508. doi: 10.1126/science.115.2993.507. [DOI] [PubMed] [Google Scholar]


