Abstract
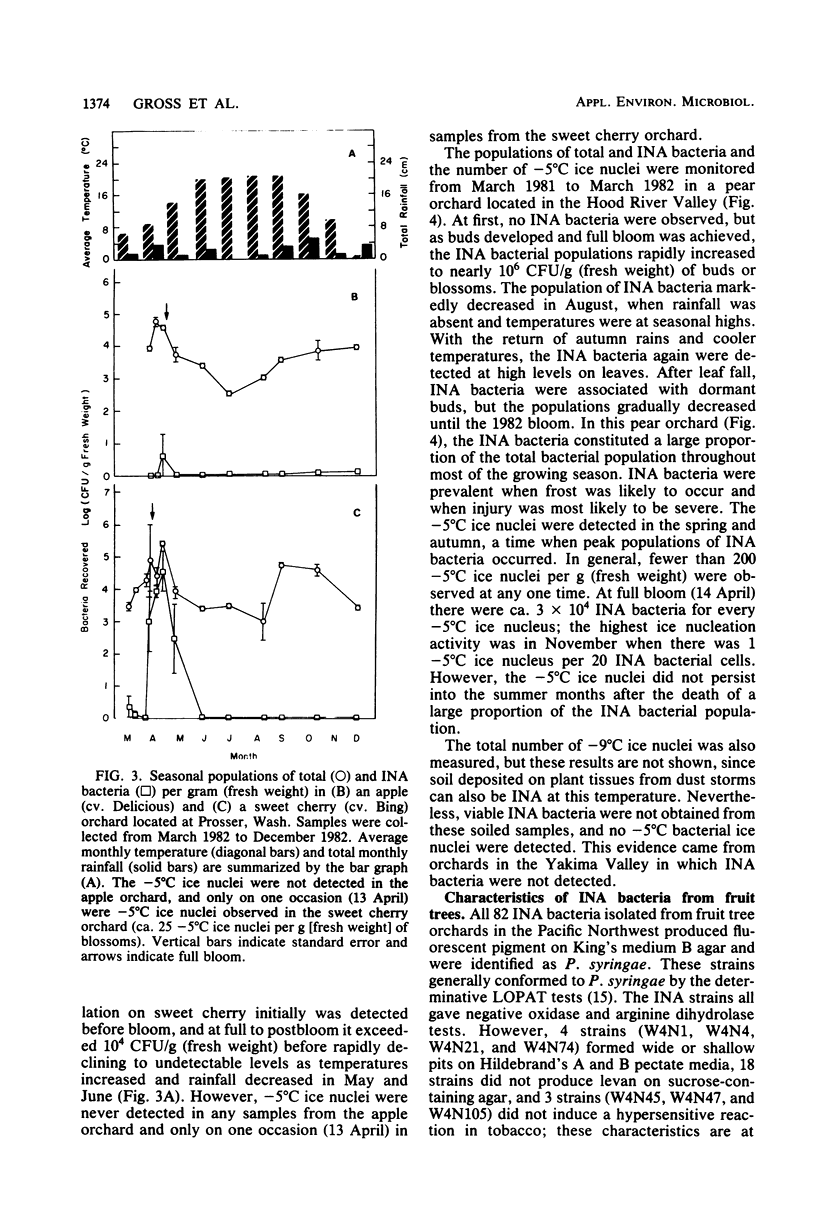
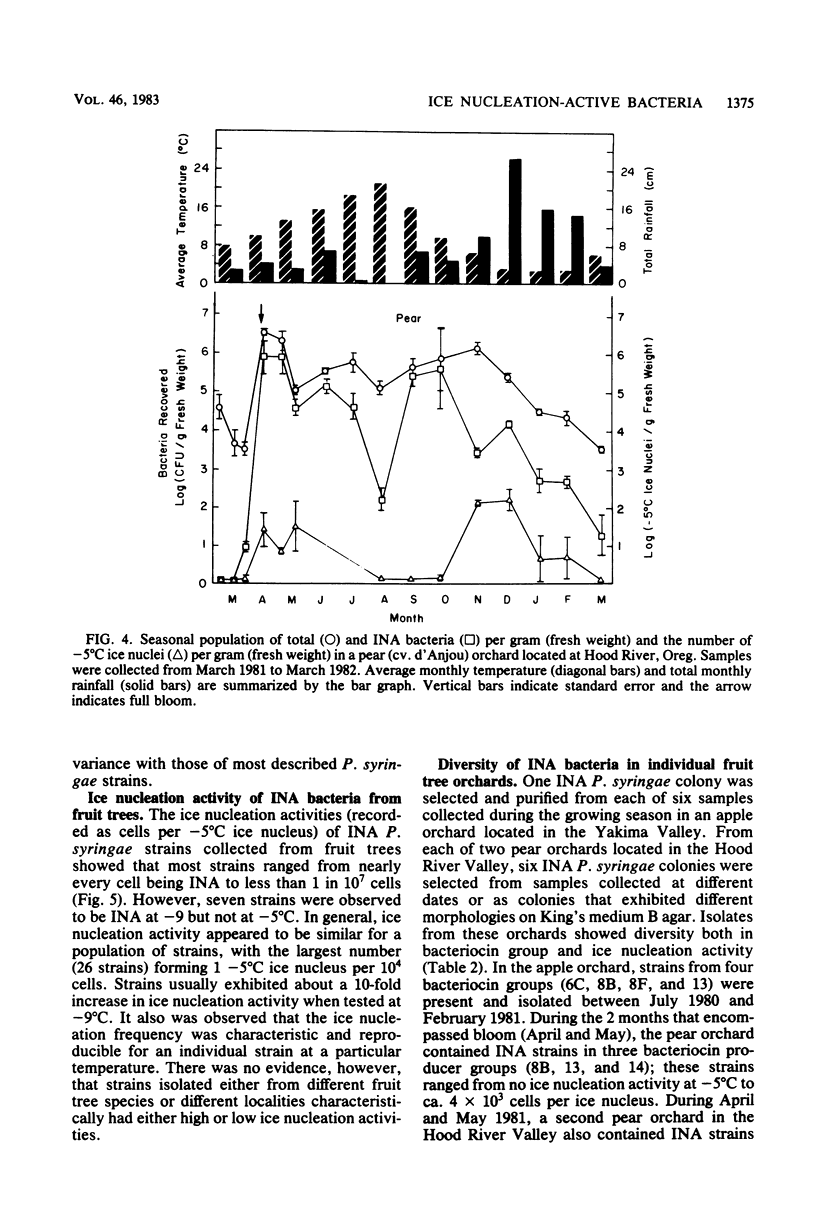
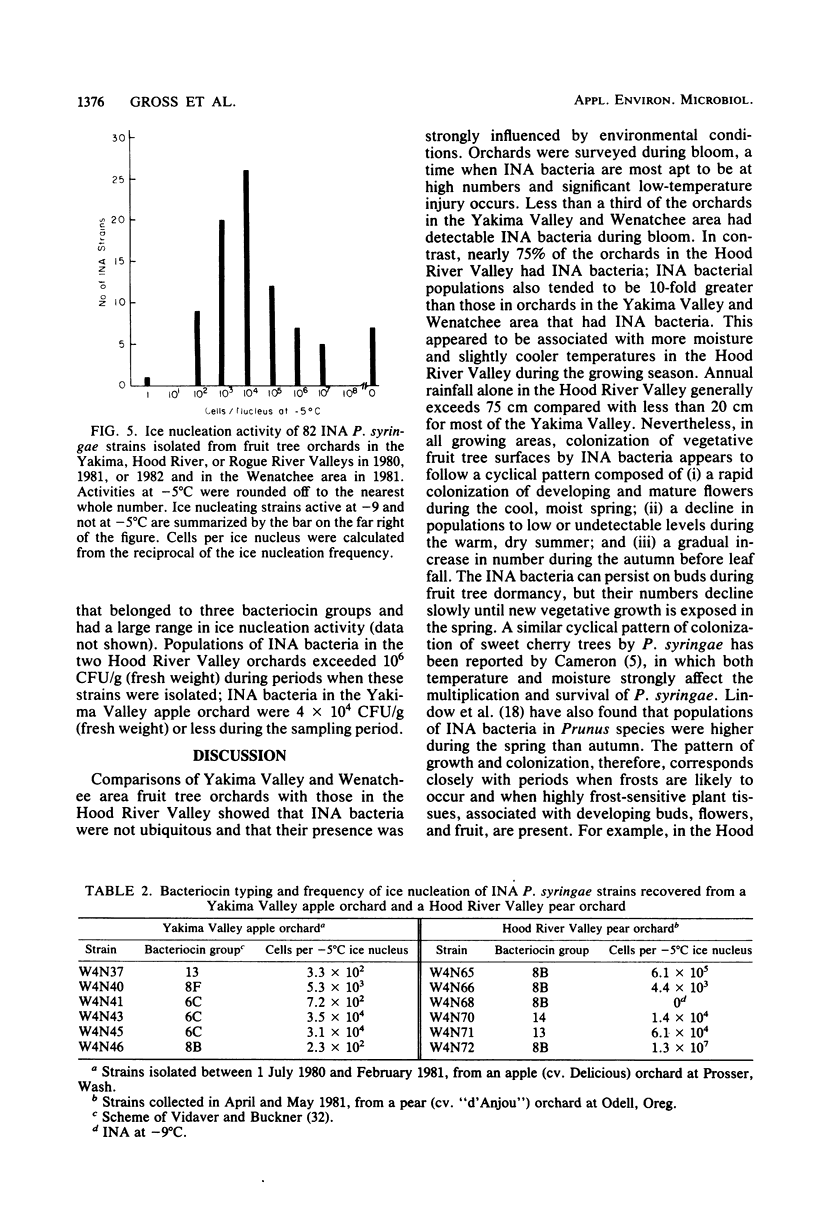
Deciduous fruit tree orchards located in the Pacific Northwest were surveyed over a 3-year period for the presence of ice nucleation-active (INA) bacteria. In the Yakima Valley, only about 30% of the fruit tree orchards contained INA bacteria (median population ca. 3 × 102 CFU/g [fresh weight]) in contrast to nearly 75% of the orchards in the Hood River Valley (median population ca. 5 × 103 CFU/g [fresh weight]). These INA populations ranged from less than 10 to over 106 CFU/g (fresh weight) of blossoms and, in Hood River Valley orchards, generally comprised over 10% of the total bacterial population. Populations of INA bacteria fluctuated during the year with highest levels developing on buds and flowers during the cool, wet spring, followed by a drop in populations during the warmer, drier, summer months and finally a gradual increase in the autumn. The INA bacteria persisted on dormant buds from which they again colonized young developing vegetative tissues. All INA bacteria were identified as Pseudomonas syringae. The frequency of ice nucleation at −5°C for these strains ranged from nearly every cell being INA to less than 1 in 107 cells. The median frequency of ice nucleation at −5°C was 104 cells per ice nucleus. The INA P. syringae strains from individual orchards were diverse with respect to bacteriocin typing and in ice nucleation frequency. The consistent absence of detectable INA bacteria or presence of low populations in most of the orchards surveyed during periods when critical temperatures (i.e., −2 to −5°C) were common indicated a limited role for INA bacteria in frost susceptibility of most Pacific Northwest orchards.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hirano S. S., Nordheim E. V., Arny D. C., Upper C. D. Lognormal distribution of epiphytic bacterial populations on leaf surfaces. Appl Environ Microbiol. 1982 Sep;44(3):695–700. doi: 10.1128/aem.44.3.695-700.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- KLEMENT Z. RAPID DETECTION OF THE PATHOGENICITY OF PHYTOPATHOGENIC PSEUDOMONADS. Nature. 1963 Jul 20;199:299–300. doi: 10.1038/199299b0. [DOI] [PubMed] [Google Scholar]
- Kazzi G. M., Gross T. L., Sokol R. J. Fetal biparietal diameter an placental grade: predictors of intrauterine growth retardation. Obstet Gynecol. 1983 Dec;62(6):755–759. [PubMed] [Google Scholar]
- Lelliott R. A., Billing E., Hayward A. C. A determinative scheme for the fluorescent plant pathogenic pseudomonads. J Appl Bacteriol. 1966 Dec;29(3):470–489. doi: 10.1111/j.1365-2672.1966.tb03499.x. [DOI] [PubMed] [Google Scholar]
- Lindemann J., Constantinidou H. A., Barchet W. R., Upper C. D. Plants as sources of airborne bacteria, including ice nucleation-active bacteria. Appl Environ Microbiol. 1982 Nov;44(5):1059–1063. doi: 10.1128/aem.44.5.1059-1063.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindow S. E., Arny D. C., Upper C. D. Bacterial ice nucleation: a factor in frost injury to plants. Plant Physiol. 1982 Oct;70(4):1084–1089. doi: 10.1104/pp.70.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindow S. E., Arny D. C., Upper C. D. Distribution of ice nucleation-active bacteria on plants in nature. Appl Environ Microbiol. 1978 Dec;36(6):831–838. doi: 10.1128/aem.36.6.831-838.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindow S. E., Hirano S. S., Barchet W. R., Arny D. C., Upper C. D. Relationship between Ice Nucleation Frequency of Bacteria and Frost Injury. Plant Physiol. 1982 Oct;70(4):1090–1093. doi: 10.1104/pp.70.4.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki L. R., Galyan E. L., Chang-Chien M. M., Caldwell D. R. Ice nucleation induced by pseudomonas syringae. Appl Microbiol. 1974 Sep;28(3):456–459. doi: 10.1128/am.28.3.456-459.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills A., Satin A. Measuring the outcome of contact tracing. 2. The responsibilities of the health worker and the outcome of contact investigations. Br J Vener Dis. 1978 Jun;54(3):192–198. doi: 10.1136/sti.54.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Vidaver A. K., Buckner S. Typing of fluorescent phytopathogenic pseudomonads by bacteriocin production. Can J Microbiol. 1978 Jan;24(1):14–18. doi: 10.1139/m78-003. [DOI] [PubMed] [Google Scholar]
- Vidaver A. K., Mathys M. L., Thomas M. E., Schuster M. L. Bacteriocins of the phytopathogens Pseudomonas syringae, P. glycinea, and P. phaseolicola. Can J Microbiol. 1972 Jun;18(6):705–713. doi: 10.1139/m72-113. [DOI] [PubMed] [Google Scholar]
- Vidaver A. K. Synthetic and complex media for the rapid detection of fluorescence of phytopathogenic pseudomonads: effect of the carbon source. Appl Microbiol. 1967 Nov;15(6):1523–1524. doi: 10.1128/am.15.6.1523-1524.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. M., Luketina R. C., Marshall A. M. The effects on temperature on growth in vitro of Pseudomonas syringae and and Xanthomonas pruni. J Appl Bacteriol. 1977 Jun;42(3):345–354. doi: 10.1111/j.1365-2672.1977.tb00702.x. [DOI] [PubMed] [Google Scholar]


